What is an ion
An Ion is simply an atom with a charge. In order to explain what is an ion, we will briefly review the atom structure. Electron configuration teaches us that an atom is more stable when its outermost orbital has a whole set of electrons. Actually, the tendency of an atom to achieve a complete outer most orbital is very powerful that it will overcome the electrostatic force that hold the atom together.
Like the case of the Fluorine atom, it will accept an extra electron to achieve a full outmost orbital even
though it will end up having  one more electrons than protons. Basically, a neutral atom has the same number of protons and electrons.
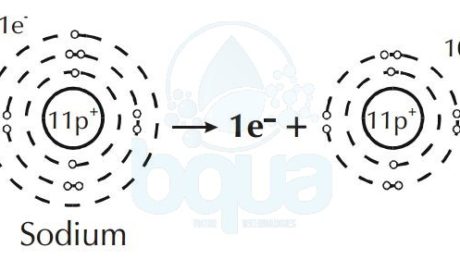
Furthermore, an element like Sodium will give up an electron to attain a full outer orbital. This results in a difference and unbalance between electrons and protons in the same atom.
When in an atom protons (+ve) are more than electrons (-ve) = result is positive ion (cation).
Sodium atom loses one electron become Sodium positive ion cation
Some atoms give up more than one electron to have a total outermost orbital. While other atoms will acquire more than one electron in order to fill the outer orbital. Since electrons have a negative charge (negative ion) and protons have a positive charge (positive ion), a difference in the number of electrons and protons results a net charge. This is when an atom is therefore called an ion.
Depending upon whether it gains or loses an electron, an ion will have either a positive or a negative
charge. Cations are ions with a net positive charged and anions are ions with a net negative charge. We know that opposite charges are attracted while same charges are repelled. That means that cations and anions are attracted to one another. The strength of the attraction may make ions form a solid called a crystal lattice.
- Published in Water Chemistry, Water Treatment
What is a molecule?
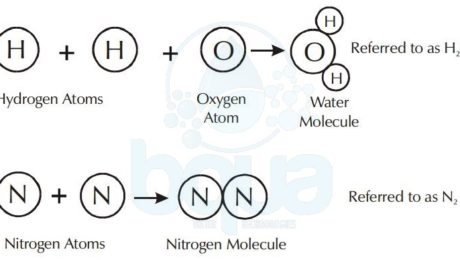
A molecule is a compound that was a substance formed by the chemical bonding of elements. Like an atom is the smallest particle of an element, the smallest discrete particle of a compound is a molecule.
A molecule is made up of atoms from one or more elements. For example, the water molecule consists of two atoms of hydrogen chemically bonded to one oxygen atom. The nitrogen molecule consists of two nitrogen atoms bonded together.
Water Compound formed from Hydrogen Atoms and Oxygen Atoms
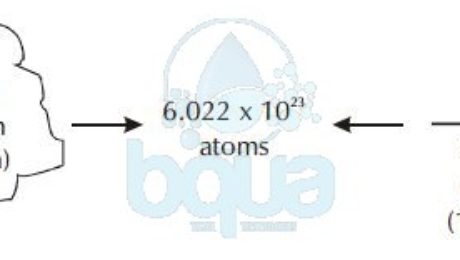
One mole equals 6.022×10^23 atoms (602,200,000,000,000,000,000,000 atoms). It is also called Avogadro Number or Avogadro Constant for the man credited for the number. Remember, one mole, or one atomic weight, or one molecular weight, or one Avogadro’s Number of any substance always contains 6.022×10^23 atoms, or molecules of that substance.
In the case of atoms, we describe the weight of one mole; the gram atomic weight. In the case of molecules, we describe it as the gram molecular weight.
Molecules are described by chemical formulas which use the chemical symbols found on the periodic table. Along with numbers to describe the number of atoms of that particular element found in the molecule.
Molecule Name Composition:
– CaSO4 – Gypsum, Calcium Sulfate – One atom of calcium, one sulfur, and four of oxygen.
– NaHCO3 – Baking Soda, Sodium Bicarbonate – One atom of sodium, one atom of hydrogen, one atom of carbon, and three atoms of oxygen.
– Al2(SO4)3 – Alum, Aluminum Sulfate – Two atoms of aluminum, three atoms of sulfur, and twelve oxygen.
– H2S – “Rotten Egg Gas”, Hydrogen Sulfide – Two hydrogens, and one atom of sulfur.
- Published in Water Chemistry, Water Treatment
What is Atomic Weight
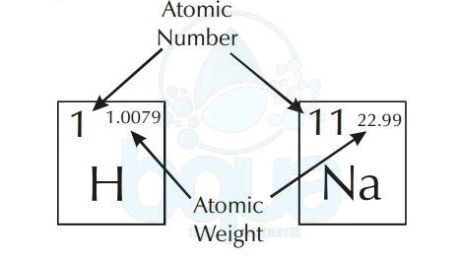
The atomic weight is simply the weight of one mole (6.022×10^23 atoms) of an element. The periodic table of elements shows the atomic weight on the upper right. The picture below shows an example of Hydrogen and Sodium atoms. Atomic weight of Hydrogen is 1.0079 when rounded to the nearest tenth = 1.0 gram. Same with Sodium atom, atomic weight is equal to 23.0 grams. For a molecule, to calculate the molecular weight we add the sum of atomic weights of the atoms in molecule.
atomic number and atomic weight in periodic table of elements
On the other hand, a gram atomic weight is the weight of one mole of an element, equal to its weight expressed in grams.
- Published in Water Chemistry, Water Treatment
What is a Functional Group
Molecular compounds owe their individual properties to functional group. These are combinations of atoms which may appear in various parts of the molecule. A list of major functional group listed below.
| Illustrative Example | Name of Functional Class | Functional Group |
| CH3CH2CH3 | Alkanes | C-C |
| CH3CH=CH2 | Alkenes | C=C |
| CH3CH/CH | Alkynes | C/C |
| CH3CH2CH2OH | Alcohols | C–OH |
| Aldehydes | ||
| Ketones | ||
| Acids | ||
| CH3CH2CH2NH2 | Amines | C-NH2 |
| CH3OCH2CH3 | Ethers | -C-O-C- |
| CH3CH2CH2Br | Halides | C-Br |
The difference in electronegativity of two bonding atoms determines if the bond will be polar or nonpolar (read about polarity). The bond between two identical atoms is always nonpolar, (for example: C-C, C=C, C=C; H-H, O=O, etc…). Bonds between atoms with electronegativities which are nearly the same are nonpolar (for example C-H). Bonds between atoms with electronegativities which are significantly different (commonly 0.9) are polar (for example, C-O, H-O, C=O, and N-H).
One molecule, especially a large complex molecule, may have more than one type of functional group. One specific functional group may be repeated several times.
A functional group may alter the properties of a compound such as melting point, boiling point, solid structure, and solubility. A functional group on an organic molecule also contribute to its hydrophobic and hydrophilic properties. Surfaces composed of nonpolar molecules will be hydrophobic. Surfaces composed of compounds containing polar bonds will be hydrophilic. You can also read about surface tension.
- Published in Water Chemistry, Water Treatment
What is a mole definition
Atoms are very small. In order to find a unit to measure an atom; scientists decided to use a unit consisting of a large group of atoms while quantifying them. The unit used for atomic measurement is called a mole.
A particular number of atoms of each element is equal to a mole of this element. A unit of measurement of the weight of a mole of atoms is called the gram atomic weight of the element and it is expressed in grams. On the other hand, one mole of a specific molecule is a gram molecular weight of this molecule.
You can find the atomic weight number in the upper right hand corner of the element box on the periodic table of elements. It basically expresses the weight of one mole of this particular element in grams.
For example, one mole of the hydrogen atom weighs 1.0079 grams. Since numbers are generally rounded off to the nearest tenth of a gram, one mole of hydrogen atoms weighs 1.0 gram. While a weight of one mole of Carbon is 12.0 grams.
atomic measurement what is a mole definition avogadro number
Avogadro Number is one mole
A mole is equal to 6.022×10^23 atoms (602,200,000,000,000,000,000,000 atoms). It is also called Avogadro Number for the man who first acknowledged the number.
One mole, or one atomic weight, or one molecular weight, or one Avogadro’s Number of any substance always contains 6.022 x 10^23 atoms, or molecules of that substance, not necessarily atoms.
- Published in Water Chemistry, Water Treatment
Molecular Weight
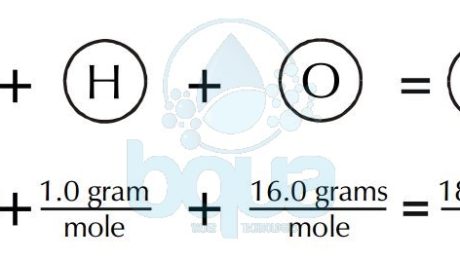
A molecular weight is simply the weight of one mole (6.022×10^23 molecules) of a compound. Like the gram atomic weight, in the case of molecules, we describe the weight of one mole (6.022 x 10^23 molecules) as the gram molecular weight. To calculate the gram molecular weight of a molecule, we add the gram atomic weight of each atom comprising the molecule. For example, to calculate the gram molecular weight of water (H2O), we would add the gram atomic weight of oxygen plus two times the gram atomic weight of hydrogen.
An example of H2O water molecule: The molecular weight is equal to the sum of atomic weights of Hydrogen and Oxygen atoms. Since there are two Hydrogen atoms and one Oxygen. Total is equal to 2(1.0 gram of Hydrogen) + 1(16.0 grams of Oxygen) = 2.0 + 16.0 = 18.0 grams molecular weight. It is also the weight of one mole of water.
Water Compound formed from Hydrogen Atoms and Oxygen Atoms
gram molecular weight of water molecule
Reverse Osmosis membranes will reject ions because of their charge. The membranes will also reject non-charged compounds (like organics) based upon molecular size. Generally, any organic less than 100 molecular weight will not be well rejected. Rejection of organics also depends upon the geometry of the molecule.
Molecular weight is important in organic chemistry since most organic molecules are quite large. Some polymers can have molecular weights in the millions.
- Published in Water Chemistry, Water Treatment
What is Surface Tension
Surface tension is one of the properties of water that is created by the Hydrogen bond. Surface tension is the reason a water droplet can have different shapes on different surfaces. As we all noticed, when we fill a glass of water to the top, water level may actually be above the glass rim. The explanation for this occurrence is the high surface tension of water on a hydrophilic surface like glass due to hydrogen bond.
surface tension of water on plastic hydrophobic
surface tension of water droplet on hydrophilic glass
Hydrophobic and Hydrophilic Examples
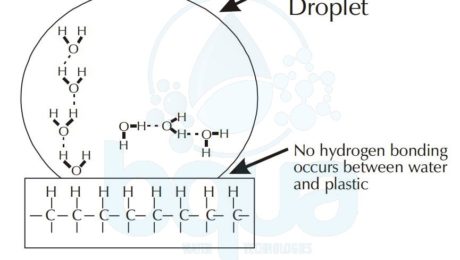
On the other hand, a water droplet tends to form a sphere like shape on a hydrophobic surface like for example plastic. Therefore, a drop of water will tend to spread on a clean glass surface. To simply explain why this happens, we will analyse the phenomenon on a molecular level. In the example of plastic and since it is a nonpolar substance; water will tend form hydrogen bonds with itself. In the example of glass which is polar; water molecules will form hydrogen bonds with the silicon dioxide of the glass. And this is what makes water tend to spread on the glass surface; attraction to glass. Read more about polarity.
Another example explaining surface tension, using a small glass tube called a capillary tube. If we place the end tip of this tube in a glass of water, water will drawn. This is because of the hydrogen bond occurring between the SiO2 of the glass tube and the Oxygen atoms in the water molecules. The definition of hydrophilic which is what describes the glass in this situation means water loving. While definition for Hydrophobic which is plastic in the above example means water hating.
surface tension capillary tube example water glass hydrophilic
A unit of measurement for the surface tension of water is dynes/cm at room temperature. Which is the force you have to overcome in order to break the surface tension of a water droplet (1cm in length). The lower the temperature the less surface tension. Hot water makes a better cleaning solution since it is considered a better wetting agent. This is due to low surface tension of hot water which allows it to better react with detergents which are hydrocarbons with a polar end called a functional group.
- Published in Water Chemistry, Water Treatment
Chemical Reaction Definition
A Chemical Reaction is the change that occur to a chemical compound or molecule to form another. This usually leads to a change in the arrangement of electrons and breaking of bonds between atoms or molecules. In addition, chemical reaction produces no change to the cores of the atoms involved. New formed substances have different characteristics and properties.
We know how atoms bond together to form molecules, but you may ask why do certain molecules form. Certain elements combine to form compounds or why do certain compounds react with one another to form new compounds.
Chemical Reaction Examples
For example, when we mix calcium carbonate and hydrochloric acid, we get a violent chemical reaction which releases a large amount of gas.
CaCO3 + 2HCl ——> CaCl2 + H2O + CO2
Calcium Carbonate + Hydrochloric Acid -> Calcium Chloride + Water + Carbon Dioxide
In fact, this equation presents a chemical reaction. In this chemical reaction calcium carbonate reacts with hydrochloric acid to form calcium chloride, water and carbon dioxide. Consequently, this along with many other chemical reactions, are considered a spontaneous chemical reaction. Hence, it will begin and continue to completion on its own.
Types of Chemical Reactions
Spontaneous Reactions
2Na + 2H2O ——> 2NaOH + H2
Metallic Sodium + Water -> Sodium Hydroxide + Hydrogen Gas
NaHSO3 + HOCl ——> NaCl + H2SO4
Sodium Bisulfite + Hypochlorous Acid -> Sodium Chloride + Sulfuric Acid
Other chemical reactions are not spontaneous, however, will still continue on its own once started with a type of energy input.
Chemical Reaction Requiring An Initial Energy Input
2H2 + O2 —Energy—>Â 2H2O
Hydrogen Gas + Oxygen Gas -Energy-> Water
CH4Â + 2O2 —Energy—>Â CO2 + 2H2O
Methane + Oxygen -Energy-> Carbon Dioxide + Water
Much as a spontaneous chemical reaction, another type of chemical reaction requiring a continuous energy input.
Chemical Reaction Requiring AÂ Continuous Energy Input
N2 + 3H2 —Cont. Energy—>Â 2NH3
Nitrogen Gas + Hydrogen Gas -Cont. Energy-> Ammonia
CaCO3 —Cont. Energy—>Â CaO + CO2
Calcium Carbonate -Cont. Energy-> Calcium Oxide + Carbon Dioxide
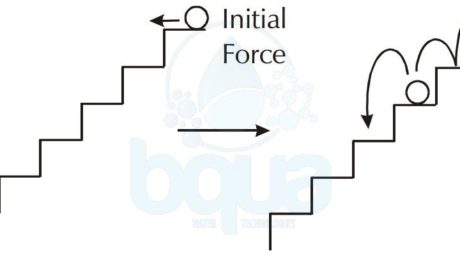
In addition, can actually explain the chemical reaction types using the example of a ball rolling down stairs. In the case of the spontaneous chemical reaction, we can see the ball rolling down a set of stairs where each step is slightly sloped downhill. What happens is that the ball continues to roll down from step to step with no assistance.
spontaneous chemical reaction example ball rolling down set of inclined stairs
As in the case of a reaction requiring an initial energy input, we can use a ball rolling down a set of stairs where each step is a level. The ball continues to roll from step to step by an initial input trigger which is a push.
chemical reaction initial energy input example ball rolling down stairs
In the last example, a chemical reaction requiring continuous energy input, we can imagine a ball starting from the bottom of the stairs. In order to get the ball to the top, we have to continually add energy to the reaction.
chemical reaction continuous energy input example ball from bottom to top of stairs
As in the case with the ball and stairs, a spontaneous chemical reaction will proceed in the direction
which produces products having the lowest potential energy level. Furthermore, the chemical reactions which require only an initial energy boost have an energy “hump†which must be overcome before the reaction will proceed on its own.
Finally, the non-spontaneous chemical reaction produces products having a higher potential energy level. Therefore, these reactions require a continuous energy input (rolling the ball uphill). The change in energy between the reactants and products of a reaction is referred to as the change in enthalpy.
- Published in Water Chemistry, Water Treatment
What is a Hydrogen Bond
A water molecule will form a bond called a hydrogen bond. A hydrogen bond is formed because of the polarity of the molecule and the fact that hydrogen atoms are present in the molecule. water molecules will form hydrogen bond with another molecule(s) of water. These bonds are not nearly as strong as the covalent bond between the hydrogen atoms and the oxygen atom within one H2O molecule.
We can simply say that a Hydrogen Bond is a weak bond that occurs between a proton of a molecule and an electronegative atom of another molecule as a result of an electrostatic attraction between the two molecules. The Hydrogen bond can either happen between two different molecules or within the same molecule.
Hydrogen bond gives water many of its amazing properties, including:
– High Boiling Point
–Â Surface Tension
– Capillary Action
– Solvent Ability
The molecular weight of water is 18; which is the sum of the atomic weights of the atoms in one molecule. Most compounds of such a small molecular weight (MW) are gases at room temperature and pressure.
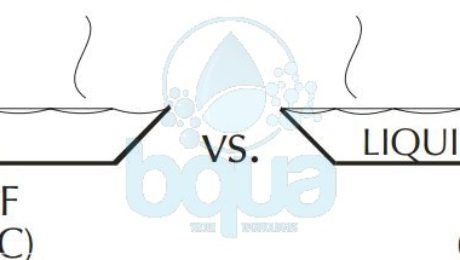
Considering the example of nitrogen (N2) as compared to water. Nitrogen has a MW of 28 and a boiling point of 321°F (-196°C) below zero. Water, on the other hand, has a MW of 18 and a boiling point of 212°F (100°C), a difference of 533°F (296°C).
hydrogen bond water and nitrogen difference properties
The difference between nitrogen and water is that the nitrogen molecule has no force of attraction between molecules. Water, of course, has the hydrogen bond between molecules. Although the hydrogen bonds are weak compared to other bonds, they are significant enough to create the tremendous difference in boiling points between the two compounds. The picture below shows few molecules of water and molecules of nitrogen within a pure liquid state.
hydrogen bonding water nitrogen difference in properties
- Published in Water Chemistry, Water Treatment
What is Polarity Definition
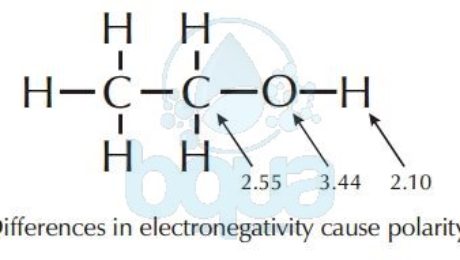
Polarity is basically the difference in electronegativity between two molecules; which is simply the affinity of an atom to electrons. Atoms of the same molecule have influence on each other in many ways. One of the reasons is electronegativity. An example is a water molecule, because of the electronegativity difference between the Oxygen atom (3.4) and Hydrogen atom (2.2) it has areas of partial negative and positive charges. Meaning that one side of the bond possesses a partial negative charge, while the other side has a partial positive charge. This is referred to as polarity, and the water molecule is said to be polar. This polarity is due to the electronegativity of the atom-of-oxygen.
The example of the Oxygen – atom in the water – molecule explains polarity. Oxygen atoms attracts electrons more than the Hydrogen atoms. This results that the pair of electrons shared spend more time around the oxygen nucleus than around the hydrogen nucleus. When a shared electrons’ pair is orbiting the oxygen nucleus, the proton of the hydrogen nucleus is exposed and the oxygen/atom has more electrons than protons. This creates the partial -ve and partial +ve charges making the molecule of water acquire polarity characteristics.
Electronegativity of oxygen cause polarity in water-molecule
The relative electronegativities of the atoms involved is what determines the polarity of bonds determined. These electronegativity values are also determined from a variation of the periodic table of the elements called the periodic table of elements.
Most hydrocarbons (molecules made up of both Carbon and Hydrogen atoms) are extremely nonpolar because of the similarity in the electronegativity of Carbon – Hydrogen. In other words, the hydrogen and carbon atoms share electrons fairly equally.
Polarity caused by electronegativity difference between two atoms in moleculeÂ
Polarity and properties of molecules
Nonpolar molecules do not have significant partial charges, therefore they will not attract polar molecules. Opposite charges attract, similar charges repel one another, but there is no attraction or repulsion between nonpolar molecules. For example, we know that gasoline (a hydrocarbon) will not mix with water. However, if we add oxygen to the hydrocarbon molecules we see that the molecules become polar. Ethanol (drinking alcohol), for instance, completely mixes with water.
Soaps and detergents are unique molecular combinations of polar and nonpolar compounds. Nonpolar part of molecule will react with oils found on soiled hands or clothing while polar part reacts with water. The result is oils removal (nonpolar) from water (high polarity). The polar end of soap is a functional group.
soap made of hydrocarbons and polar end functional group
The incompatibility between nonpolar compounds and water is due to the attraction that water molecules exhibit for one another. Positive charge on the hydrogen of a water-molecule will attract negative charge of atom of oxygen in adjacent water molecule. This attraction, called a hydrogen bond, will cause a type of bond between the molecules themselves.
Because of lack of charges, nonpolar compound won’t have the strength to break the hydrogen bonds between the water molecules. Therefore the nonpolar compound won’t combine with water.
- Published in Water Chemistry, Water Treatment
What is an Atom Definition
An atom is the most discrete indivisible particle that forms an element. Atoms of the same element are identical; different elements have non-similar atoms. The most simple of all elements is Hydrogen.Â
Example: Hydrogen atoms are similar while Oxygen atoms are different than Hydrogen atoms. Two hydrogen atoms and one Oxygen combined form the water molecule H2O.
The atoms are extremely small. Therefore, even the most powerful microscopes cannot see them. Actually, 6.022X10^23 hydrogen atoms (Avogadro Number) weigh only one gram. There are 454 grams in one pound.
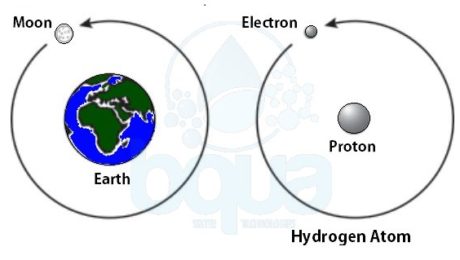
In order to understand the nature of the atom, we should identify the forces that are holding it together. Much as our plant, gravity is the force holding the moon in its orbit around the earth. Gravity is the force of attraction between two large masses.
In the case of the atom, the small size of the particles and the speed at which the electron travels would prohibit a force such as gravity from being able to hold the atom together. Scientists have learned that electrostatic force is the force that maintains the atom.
Atoms Composition
While atoms are composed of small subatomic particles; these particles are the electron, the proton and the neutron. The proton and neutron are found in the nucleus which is the center of the atom. The electron is negatively charged and orbits around the nucleus.
atom composed of subatomic particles electron proton neutron
Atoms from different elements are constituted of the same type of subatomic particles. However, the proportions of the subatomic particles are different for each element. Hydrogen for example has two subatomic particles; an electron and a proton. In the case of hydrogen, a single electron orbits a single proton. ÙAn example is the moon which orbits the earth. Same as in the case of earth, the proton of the hydrogen atom is much larger than the electron.
an electron orbits proton in nucleus in an atom like moon orbits earth
Moreover, if we add a subatomic particle to a Hydrogen atom, we create another atom of another element. Note: The number of subatomic particles is what characterizes atoms of different elements. In addition, if we want to create a Helium atom, we should only add an additional electron, an additional proton and two neutrons to the Hydrogen atom. As a result, we’ll have two electrons orbiting four other particles, two protons and two neutrons in the nucleus.
- Published in Water Chemistry, Water Treatment
What is Osmosis Definition
Osmosis is a natural process (phenomenon) that occurs in nature around us. The Osmosis process is even happening now within our bodies while reading this post.
A simple definition of Osmosis is that it is the tendency of a fluid to pass or flow through a semipermeable membrane into solution of higher concentration. Osmosis is the process whereby the water that we drink eventually ends up in our blood, muscles, and cells. The lining in our intestine is a membrane. The yolk of an egg has a membrane surrounding it that releases its contents when broken. If we simply wrap a piece of meat with plastic wrap, we can consider the plastic wrap a membrane.
A definition of permeable is when materials can pass through it. For example, a kitchen strainer or sieve is totally permeable to water. Meaning that water passes freely through it. Semipermeable means that some materials pass while others don’t. The kitchen strainer is an example. Since it allows water to pass freely through it but will not allow large solids to infiltrate.
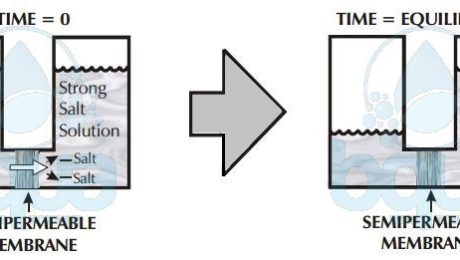
Back to Osmosis, water passes through semi-membrane from low salt concentration into a solution of high concentration. The reason is simple, let’s imagine having a salt solution and pure water separated by such a membrane. We know that water molecules may freely pass through the membrane but dissolved substances can’t. Given that all molecules are in motion, which liquid will have the greatest number of molecules colliding with the membrane? The pure water or the salt water?
Per given area of membrane, the pure clean water side will have more molecules of water that collide with the membrane than the side of water with salt. Water in Osmosis, therefore will pass from the side with pure or clean water to the salt water side. The higher the concentration of salt, the fewer the collisions of water molecules with the membrane. In Osmosis, water always passes through a semi-permeable membrane, therefore, from a lower salt concentrated side into a higher concentrated side.
osmosis: lower salt concentration to higher concentration
Osmosis then describes the tendency of fluid (water) to pass through a semi-permeable membrane, into a solution of higher concentration. The process stops when the number of water molecules colliding with either side of the membrane are equal. The number of collisions is a function of the salt concentration and the pressure on whichever membrane side. These are called osmotic pressure and applied pressure.
Osmosis reaches equilibrium when the concentration of dissolved solids at both compartments is equal. Meaning no more net flow from one side to another. The side that was the higher concentration solution however now has a higher water level than the other side due to osmosis. This difference in height between both sides is due to osmotic pressure.
Now that you learned about Osmosis, you might want to read about Reverse Osmosis (RO).
- Published in Water Chemistry, Water Treatment
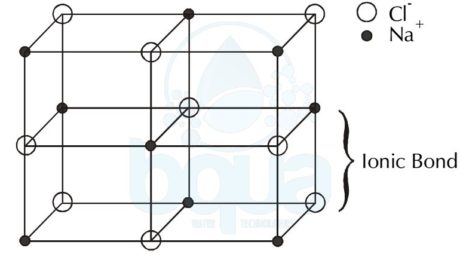
What is a Crystal Lattice ?
Crystal Lattice is the solid formed by the force of attractions between the charged ions; anions and the cations. It is basically the three dimensional pattern or arrangement of  the atoms inside the crystal.
Since cations are positively charged ions and anions are negatively charged ions, they are strongly attracted to each other. This attraction is very strong that the ions may bond together into a solid called a crystal lattice. The bond that holds the two oppositely-charged ions is called an ionic bond.
Types of Crystal Lattice Structures:
Crystal Lattice exist in seven crystal lattice structures which are:Â
- Cubic
- Monoclinic
- Triclinic
- Hexagonal
- Tetragonal
- Orthorhombic
- Rhombohedral
Each of these crystal lattice structures have unique variants which make the total number of crystal lattice structures 14 Bravais Lattices. The cubic crystal lattice structure has a symmetrical shape and is considered the simplest and most symmetrical form of crystal lattices.
The 3 types of cubic crystal lattice structures are:Â
- Simple Cubic (SC)
- Body-Centered Cubic (CBC)
- Face Centered Cubic (FCC).
Sodium Chloride Crystal Lattice:
An example is the Sodium Chloride – salt – molecule NaCl, since the Sodium (Na) became a cation when it lost an electron to give it to the Chloride atom (Cl) which hence became a negatively charged anion. The ionic bond is the force that is keeping the anions and cations in the solid shape of crystal lattice form.
crystal lattice formed by force of attraction between anions and cations ionic bond
You can also read about Molecular Geometry.
- Published in Water Chemistry, Water Treatment
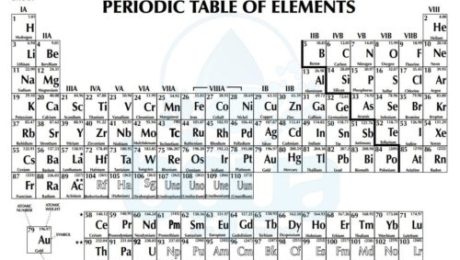
What is Periodic Table of Elements:
Atoms of different elements are distinguished only by the number of subatomic particles; electrons, protons and neutrons. Scientists decided to organize the elements in a periodic table based on the number of these particles in the atoms of each element. By the time, a chart was developed which described the atom structure of each element. This chart has come to be known as the periodic table of elements.
periodic table of elements including atomic number atomic weight
By observing the periodic table there are a lot of important information for each element. We see that each element in the periodic table has a Chemical Symbol which is usually the first one or two letters of the element’s name. For instance: H for Hydrogen, He for Helium, O for Oxygen, Ca for calcium, etc. In other cases, there are elements that have symbols which do not correspond to the name of the element. For instance: Na for sodium, K for potassium, etc. These letters are abbreviations of the Latin name of these elements. Na are the first two letters of Natrium (Sodium in Latin), K is the first letter of Kalium (Potassium in Latin) and so on.
Another observation in the periodic table is that each element has a corresponding number. This number is called the atomic number and it refers to the number of protons in the nucleus. Since we know that in neutral atoms (not charged) the number of protons is equal to the number of electrons, the atomic number also equals the number of electrons.
atomic number and atomic weight in periodic table of elements
The number which is greater than the atomic number is called the atomic weight. The atomic weight also called gram atomic weight is the weight of a particular number of atoms of the element in grams.
- Published in Water Chemistry, Water Treatment
- 1
- 2