Electron configuration:
The electron configuration is simply the orientation of electrons about the nucleus of the atom. It is known that the atom structure consists electrons, protons and neutrons. An electron has a negative charge, a proton has a positive charge and is in the nucleus with neutrons. Neutrons have neutral charges (non-charged particles).
In a neutral atom, the number of electrons that electron configuration forms are always equal to the number of protons inside the nucleus. The neutron does not contribute to the electrostatic force which is the force that holds the atom together.
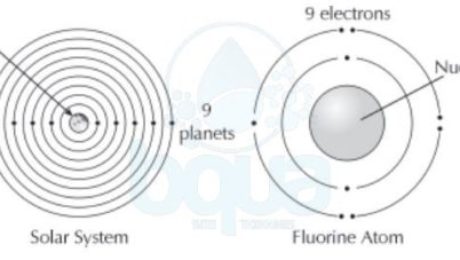
It was found that in the atom structure, electrons tend to orient themselves in a particular fashion. This orientation is called the electron configuration. When atoms get larger and gain more electrons, electrons will exist in particular orbits around the nucleus. A living example of such practice is how each planet in our solar system circles the sun in its own orbit.
electron configuration electrons orient themselves around nucleus protons earth planets
In the electron configuration, we find that more than one electron may exist in the same orbit around the nucleus. The first orbital may contain one to two electrons. While the second orbital may contain up to eight electrons. Each subsequent orbital can hold more and more electrons.
Equation for electron configuration
The equation for the number of electrons in each energy level is:
# of electrons = 2(n)^2 Â where “n” is the nth level
For example: Number of electrons in first energy level = 2(1)^2 = 2(1) = 2 electrons
While number of electrons in the second energy level = 2(2)^2 = 2(4) = 8 electrons and so on.
In the electron configuration, an atom is most stable when its outermost orbital is completely full of electrons. In other words two electrons in the first orbital makes the atom more stable than one. While 8 electrons in the second orbital makes it more stable than < 8 electrons.
atom structure more stable when outermost orbital full of electrons
The orbitals are usually illustrated in two dimensional drawings, in reality the orbitals exist in various three dimensional shapes. Each orbital contains one or more suborbitals identified as s, p, and d. The s, p, and d suborbitals are shaped differently. The shape of the suborbital does not affect the number of electrons in the orbital.
- Published in Water Chemistry, Water Treatment
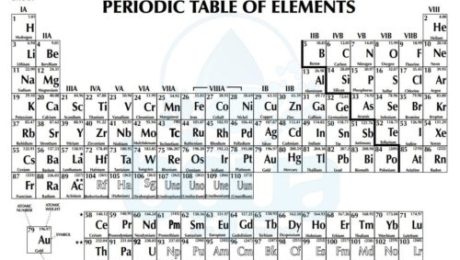
What is Periodic Table of Elements:
Atoms of different elements are distinguished only by the number of subatomic particles; electrons, protons and neutrons. Scientists decided to organize the elements in a periodic table based on the number of these particles in the atoms of each element. By the time, a chart was developed which described the atom structure of each element. This chart has come to be known as the periodic table of elements.
periodic table of elements including atomic number atomic weight
By observing the periodic table there are a lot of important information for each element. We see that each element in the periodic table has a Chemical Symbol which is usually the first one or two letters of the element’s name. For instance: H for Hydrogen, He for Helium, O for Oxygen, Ca for calcium, etc. In other cases, there are elements that have symbols which do not correspond to the name of the element. For instance: Na for sodium, K for potassium, etc. These letters are abbreviations of the Latin name of these elements. Na are the first two letters of Natrium (Sodium in Latin), K is the first letter of Kalium (Potassium in Latin) and so on.
Another observation in the periodic table is that each element has a corresponding number. This number is called the atomic number and it refers to the number of protons in the nucleus. Since we know that in neutral atoms (not charged) the number of protons is equal to the number of electrons, the atomic number also equals the number of electrons.
atomic number and atomic weight in periodic table of elements
The number which is greater than the atomic number is called the atomic weight. The atomic weight also called gram atomic weight is the weight of a particular number of atoms of the element in grams.
- Published in Water Chemistry, Water Treatment