Electron configuration:
The electron configuration is simply the orientation of electrons about the nucleus of the atom. It is known that the atom structure consists electrons, protons and neutrons. An electron has a negative charge, a proton has a positive charge and is in the nucleus with neutrons. Neutrons have neutral charges (non-charged particles).
In a neutral atom, the number of electrons that electron configuration forms are always equal to the number of protons inside the nucleus. The neutron does not contribute to the electrostatic force which is the force that holds the atom together.
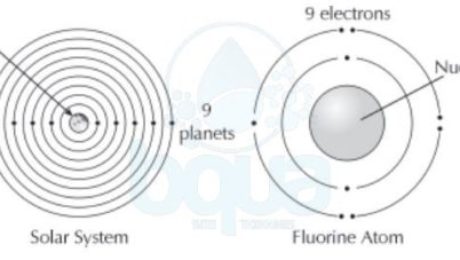
It was found that in the atom structure, electrons tend to orient themselves in a particular fashion. This orientation is called the electron configuration. When atoms get larger and gain more electrons, electrons will exist in particular orbits around the nucleus. A living example of such practice is how each planet in our solar system circles the sun in its own orbit.
electron configuration electrons orient themselves around nucleus protons earth planets
In the electron configuration, we find that more than one electron may exist in the same orbit around the nucleus. The first orbital may contain one to two electrons. While the second orbital may contain up to eight electrons. Each subsequent orbital can hold more and more electrons.
Equation for electron configuration
The equation for the number of electrons in each energy level is:
# of electrons = 2(n)^2 Â where “n” is the nth level
For example: Number of electrons in first energy level = 2(1)^2 = 2(1) = 2 electrons
While number of electrons in the second energy level = 2(2)^2 = 2(4) = 8 electrons and so on.
In the electron configuration, an atom is most stable when its outermost orbital is completely full of electrons. In other words two electrons in the first orbital makes the atom more stable than one. While 8 electrons in the second orbital makes it more stable than < 8 electrons.
atom structure more stable when outermost orbital full of electrons
The orbitals are usually illustrated in two dimensional drawings, in reality the orbitals exist in various three dimensional shapes. Each orbital contains one or more suborbitals identified as s, p, and d. The s, p, and d suborbitals are shaped differently. The shape of the suborbital does not affect the number of electrons in the orbital.
- Published in Water Chemistry, Water Treatment
What is an ion
An Ion is simply an atom with a charge. In order to explain what is an ion, we will briefly review the atom structure. Electron configuration teaches us that an atom is more stable when its outermost orbital has a whole set of electrons. Actually, the tendency of an atom to achieve a complete outer most orbital is very powerful that it will overcome the electrostatic force that hold the atom together.
Like the case of the Fluorine atom, it will accept an extra electron to achieve a full outmost orbital even
though it will end up having  one more electrons than protons. Basically, a neutral atom has the same number of protons and electrons.
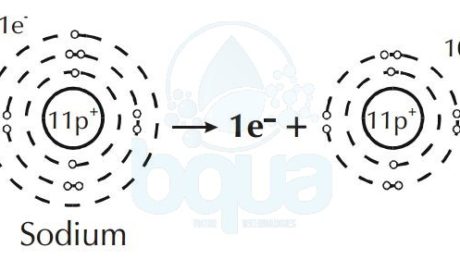
Furthermore, an element like Sodium will give up an electron to attain a full outer orbital. This results in a difference and unbalance between electrons and protons in the same atom.
When in an atom protons (+ve) are more than electrons (-ve) = result is positive ion (cation).
Sodium atom loses one electron become Sodium positive ion cation
Some atoms give up more than one electron to have a total outermost orbital. While other atoms will acquire more than one electron in order to fill the outer orbital. Since electrons have a negative charge (negative ion) and protons have a positive charge (positive ion), a difference in the number of electrons and protons results a net charge. This is when an atom is therefore called an ion.
Depending upon whether it gains or loses an electron, an ion will have either a positive or a negative
charge. Cations are ions with a net positive charged and anions are ions with a net negative charge. We know that opposite charges are attracted while same charges are repelled. That means that cations and anions are attracted to one another. The strength of the attraction may make ions form a solid called a crystal lattice.
- Published in Water Chemistry, Water Treatment