What is an ion
An Ion is simply an atom with a charge. In order to explain what is an ion, we will briefly review the atom structure. Electron configuration teaches us that an atom is more stable when its outermost orbital has a whole set of electrons. Actually, the tendency of an atom to achieve a complete outer most orbital is very powerful that it will overcome the electrostatic force that hold the atom together.
Like the case of the Fluorine atom, it will accept an extra electron to achieve a full outmost orbital even
though it will end up having  one more electrons than protons. Basically, a neutral atom has the same number of protons and electrons.
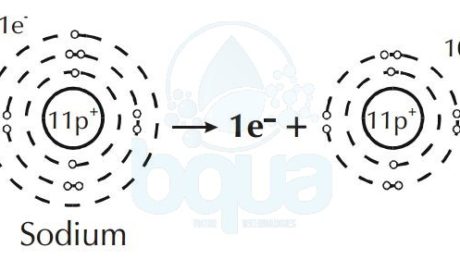
Furthermore, an element like Sodium will give up an electron to attain a full outer orbital. This results in a difference and unbalance between electrons and protons in the same atom.
When in an atom protons (+ve) are more than electrons (-ve) = result is positive ion (cation).
Sodium atom loses one electron become Sodium positive ion cation
Some atoms give up more than one electron to have a total outermost orbital. While other atoms will acquire more than one electron in order to fill the outer orbital. Since electrons have a negative charge (negative ion) and protons have a positive charge (positive ion), a difference in the number of electrons and protons results a net charge. This is when an atom is therefore called an ion.
Depending upon whether it gains or loses an electron, an ion will have either a positive or a negative
charge. Cations are ions with a net positive charged and anions are ions with a net negative charge. We know that opposite charges are attracted while same charges are repelled. That means that cations and anions are attracted to one another. The strength of the attraction may make ions form a solid called a crystal lattice.
- Published in Water Chemistry, Water Treatment
What is a Functional Group
Molecular compounds owe their individual properties to functional group. These are combinations of atoms which may appear in various parts of the molecule. A list of major functional group listed below.
| Illustrative Example | Name of Functional Class | Functional Group |
| CH3CH2CH3 | Alkanes | C-C |
| CH3CH=CH2 | Alkenes | C=C |
| CH3CH/CH | Alkynes | C/C |
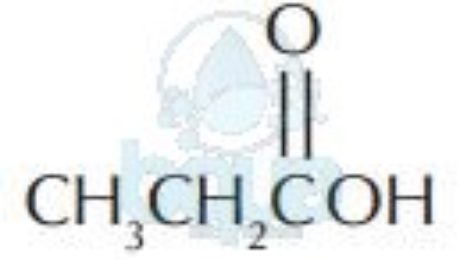
| CH3CH2CH2OH | Alcohols | C–OH |
| Aldehydes | ||
| Ketones | ||
| Acids | ||
| CH3CH2CH2NH2 | Amines | C-NH2 |
| CH3OCH2CH3 | Ethers | -C-O-C- |
| CH3CH2CH2Br | Halides | C-Br |
The difference in electronegativity of two bonding atoms determines if the bond will be polar or nonpolar (read about polarity). The bond between two identical atoms is always nonpolar, (for example: C-C, C=C, C=C; H-H, O=O, etc…). Bonds between atoms with electronegativities which are nearly the same are nonpolar (for example C-H). Bonds between atoms with electronegativities which are significantly different (commonly 0.9) are polar (for example, C-O, H-O, C=O, and N-H).
One molecule, especially a large complex molecule, may have more than one type of functional group. One specific functional group may be repeated several times.
A functional group may alter the properties of a compound such as melting point, boiling point, solid structure, and solubility. A functional group on an organic molecule also contribute to its hydrophobic and hydrophilic properties. Surfaces composed of nonpolar molecules will be hydrophobic. Surfaces composed of compounds containing polar bonds will be hydrophilic. You can also read about surface tension.
- Published in Water Chemistry, Water Treatment
What is Polarity Definition
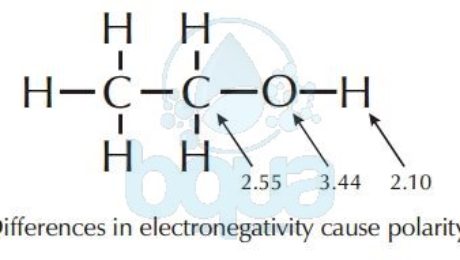
Polarity is basically the difference in electronegativity between two molecules; which is simply the affinity of an atom to electrons. Atoms of the same molecule have influence on each other in many ways. One of the reasons is electronegativity. An example is a water molecule, because of the electronegativity difference between the Oxygen atom (3.4) and Hydrogen atom (2.2) it has areas of partial negative and positive charges. Meaning that one side of the bond possesses a partial negative charge, while the other side has a partial positive charge. This is referred to as polarity, and the water molecule is said to be polar. This polarity is due to the electronegativity of the atom-of-oxygen.
The example of the Oxygen – atom in the water – molecule explains polarity. Oxygen atoms attracts electrons more than the Hydrogen atoms. This results that the pair of electrons shared spend more time around the oxygen nucleus than around the hydrogen nucleus. When a shared electrons’ pair is orbiting the oxygen nucleus, the proton of the hydrogen nucleus is exposed and the oxygen/atom has more electrons than protons. This creates the partial -ve and partial +ve charges making the molecule of water acquire polarity characteristics.
Electronegativity of oxygen cause polarity in water-molecule
The relative electronegativities of the atoms involved is what determines the polarity of bonds determined. These electronegativity values are also determined from a variation of the periodic table of the elements called the periodic table of elements.
Most hydrocarbons (molecules made up of both Carbon and Hydrogen atoms) are extremely nonpolar because of the similarity in the electronegativity of Carbon – Hydrogen. In other words, the hydrogen and carbon atoms share electrons fairly equally.
Polarity caused by electronegativity difference between two atoms in moleculeÂ
Polarity and properties of molecules
Nonpolar molecules do not have significant partial charges, therefore they will not attract polar molecules. Opposite charges attract, similar charges repel one another, but there is no attraction or repulsion between nonpolar molecules. For example, we know that gasoline (a hydrocarbon) will not mix with water. However, if we add oxygen to the hydrocarbon molecules we see that the molecules become polar. Ethanol (drinking alcohol), for instance, completely mixes with water.
Soaps and detergents are unique molecular combinations of polar and nonpolar compounds. Nonpolar part of molecule will react with oils found on soiled hands or clothing while polar part reacts with water. The result is oils removal (nonpolar) from water (high polarity). The polar end of soap is a functional group.
soap made of hydrocarbons and polar end functional group
The incompatibility between nonpolar compounds and water is due to the attraction that water molecules exhibit for one another. Positive charge on the hydrogen of a water-molecule will attract negative charge of atom of oxygen in adjacent water molecule. This attraction, called a hydrogen bond, will cause a type of bond between the molecules themselves.
Because of lack of charges, nonpolar compound won’t have the strength to break the hydrogen bonds between the water molecules. Therefore the nonpolar compound won’t combine with water.
- Published in Water Chemistry, Water Treatment
What is Periodic Table of Elements:
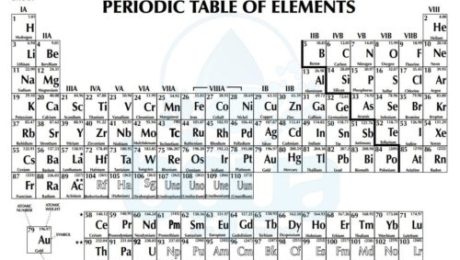
Atoms of different elements are distinguished only by the number of subatomic particles; electrons, protons and neutrons. Scientists decided to organize the elements in a periodic table based on the number of these particles in the atoms of each element. By the time, a chart was developed which described the atom structure of each element. This chart has come to be known as the periodic table of elements.
periodic table of elements including atomic number atomic weight
By observing the periodic table there are a lot of important information for each element. We see that each element in the periodic table has a Chemical Symbol which is usually the first one or two letters of the element’s name. For instance: H for Hydrogen, He for Helium, O for Oxygen, Ca for calcium, etc. In other cases, there are elements that have symbols which do not correspond to the name of the element. For instance: Na for sodium, K for potassium, etc. These letters are abbreviations of the Latin name of these elements. Na are the first two letters of Natrium (Sodium in Latin), K is the first letter of Kalium (Potassium in Latin) and so on.
Another observation in the periodic table is that each element has a corresponding number. This number is called the atomic number and it refers to the number of protons in the nucleus. Since we know that in neutral atoms (not charged) the number of protons is equal to the number of electrons, the atomic number also equals the number of electrons.
atomic number and atomic weight in periodic table of elements
The number which is greater than the atomic number is called the atomic weight. The atomic weight also called gram atomic weight is the weight of a particular number of atoms of the element in grams.
- Published in Water Chemistry, Water Treatment