What is Electronegativity – Electronegativity Definition
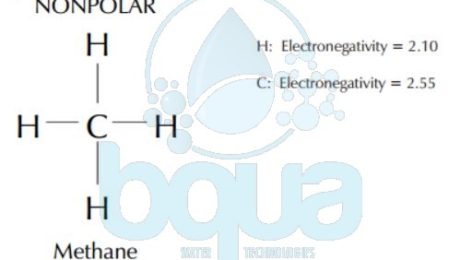
The electronegativity determines whether a bond between two atoms is polar or nonpolar which is defined by Polarity. The electronegativity is a measurement of how attractive an atom is to electrons. The more electronegative an element is, the more the shared electrons will spend around that atom. When one atom in a bond gets to keep the electrons more than the other, it assumes a partial negative charge. The other bonded atom has the electrons less often, so assumes a slight positive charge. Like the negative and positive poles of a magnet. One end of the bond is positive and one end is negative, so the bond is said to be polar. Carbon and hydrogen bonds, as in methane, are nonpolar because the electronegativity of both are very close. Carbon and oxygen bonds are polar because the electronegativity of them are substantially different.
Electronegativity example in Methane molecule
Polar substances dissolve in polar substances because the partial positive & negative charges of one molecule are attracted to the partial +ve and -ve charges of other molecules. Nonpolar substances will dissolve in nonpolar substances. This is because of other forces of attraction, such as Van der Waals’ forces, can come into play. Nonpolar compounds generally do not dissolve in polar compounds, and vice versa, because there is no attraction between things that are charged and things that aren’t. Since water is polar, compounds which are polar will generally dissolve well.
Therefore, if we have an organic which is polar, such as methyl alcohol, it will dissolve well in water. In general, the more polar an organic is, the more it will be dissolved. More importantly for this chapter, the more nonpolar a compound is, the less it will be dissolved and the more it will tend to be a suspended particle.
SIZE: One other aspect of suspended solids is their size. Very large molecules, even if they are polar, will tend to be particles. For example, we have discussed silica (SiO2) at length. If we look at the bonding between Si and O, we see that silicon has an electronegativity of 1.90 and O has an electronegativity of 3.44. Is this a polar bond? Given that water is polar and hydrogen has an electronegativity of 2.10, silica is more polar than water. Yet we see that sand is a particle and not dissolved.
To summarize, then, the degree of polarity and the size of an organic compound will determine if it is suspended or dissolved.
- Published in Water Chemistry, Water Treatment
What is a Covalent Bond
Atoms can bond by sharing electrons in their outermost orbitals and thus giving them the privilege of having a full outermost orbital. For example, the element Hydrogen exists as H2 molecule consisting of two atoms of Hydrogen atom. A covalent bond is simply the force between two atoms resulting from the sharing of electrons in the outermost orbital. Therefore a covalent bond attracts two atoms very close to each others because both atoms share the same electrons in their outermost orbitals.
hydrogen gas formed by covalent bond sharing electron between two hydrogen atoms
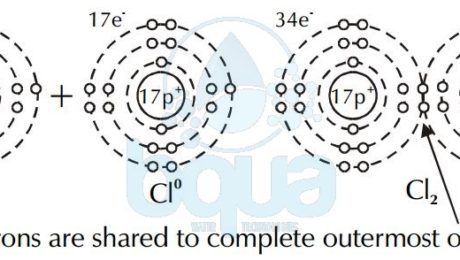
The case of Hydrogen cannot be an ionic bond. That is because Hydrogen ions each have the same charge and therefore are not attracted to one another. This is why a covalent bond happens only when two atoms have a difference in electronegativity of > or = 0.9. Instead, electrons are shared between the atoms to fill the outermost orbital. This type of bond is called a covalent bond. Two atoms of chlorine (Cl0) share a pair of electrons to form chlorine gas (Cl2).
electron shared covalent bond chlorine atom forming chlorine gas
Some molecules formed by covalent bonds may still give up or acquire one or more electrons resulting in a net positive or negative charge. These molecules are called molecular ions.
A covalent bond is very strong. The energy needed to break it is bigger than the thermal energy existing at 25°C which is room temperature. The thermal energy at 25°C is <1 kCal/mole kilo calorie per mole, while to break a typical Carbon covalent bond in an Ethane molecule we need about 83 Kilocalorie per mole.
Properties of molecules defined by covalent bond
While a central atom in a molecule attracts other atoms by covalent bonds, the bonds between these atoms are forming particular angles between them. The force of repulsion among these atoms and particularly the outermost electrons is what shape and determine the angles degrees. The angles and the shape formed is what gives the molecule its properties and shape. An example is the angle formed between Oxygen and Hydrogen atoms in a water molecule, an angle of 105°. You can read about the molecular geometry.
When atoms formed in a covalent bond have the same or very close electronegativity – typically less than 0.9 – the bond (the force of attraction) is equal between electrons since they’re almost identical. This kind of bond is called a non-polar bond. Carbon-Carbon and Carbon-Hydrogen bonds (also called Hydrocarbon) is an example on non-polar bonds.
A polar bond occurs when the difference in electronegativity between two atoms are more than or equal to 0.9. In a polar bond, there’s a partial negative charge (δ−) and a partial positive charge (δ+). This is the case in a water molecule H2O. The bond between Oxygen and Hydrogen is polar since difference in electronegativity is 1.2. (Electronegativity of Oxygen is 3.4 and Electronegativity of Hydrogen is 2.2).
- Published in Water Chemistry, Water Treatment
What is a Functional Group
Molecular compounds owe their individual properties to functional group. These are combinations of atoms which may appear in various parts of the molecule. A list of major functional group listed below.
| Illustrative Example | Name of Functional Class | Functional Group |
| CH3CH2CH3 | Alkanes | C-C |
| CH3CH=CH2 | Alkenes | C=C |
| CH3CH/CH | Alkynes | C/C |
| CH3CH2CH2OH | Alcohols | C–OH |
| Aldehydes | ||
| Ketones | ||
| Acids | ||
| CH3CH2CH2NH2 | Amines | C-NH2 |
| CH3OCH2CH3 | Ethers | -C-O-C- |
| CH3CH2CH2Br | Halides | C-Br |
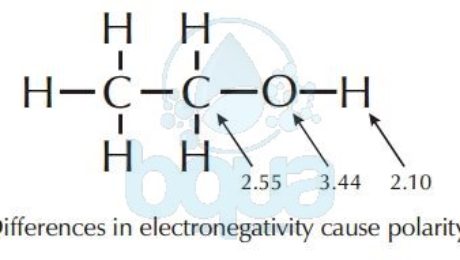
The difference in electronegativity of two bonding atoms determines if the bond will be polar or nonpolar (read about polarity). The bond between two identical atoms is always nonpolar, (for example: C-C, C=C, C=C; H-H, O=O, etc…). Bonds between atoms with electronegativities which are nearly the same are nonpolar (for example C-H). Bonds between atoms with electronegativities which are significantly different (commonly 0.9) are polar (for example, C-O, H-O, C=O, and N-H).
One molecule, especially a large complex molecule, may have more than one type of functional group. One specific functional group may be repeated several times.
A functional group may alter the properties of a compound such as melting point, boiling point, solid structure, and solubility. A functional group on an organic molecule also contribute to its hydrophobic and hydrophilic properties. Surfaces composed of nonpolar molecules will be hydrophobic. Surfaces composed of compounds containing polar bonds will be hydrophilic. You can also read about surface tension.
- Published in Water Chemistry, Water Treatment
What is Polarity Definition
Polarity is basically the difference in electronegativity between two molecules; which is simply the affinity of an atom to electrons. Atoms of the same molecule have influence on each other in many ways. One of the reasons is electronegativity. An example is a water molecule, because of the electronegativity difference between the Oxygen atom (3.4) and Hydrogen atom (2.2) it has areas of partial negative and positive charges. Meaning that one side of the bond possesses a partial negative charge, while the other side has a partial positive charge. This is referred to as polarity, and the water molecule is said to be polar. This polarity is due to the electronegativity of the atom-of-oxygen.
The example of the Oxygen – atom in the water – molecule explains polarity. Oxygen atoms attracts electrons more than the Hydrogen atoms. This results that the pair of electrons shared spend more time around the oxygen nucleus than around the hydrogen nucleus. When a shared electrons’ pair is orbiting the oxygen nucleus, the proton of the hydrogen nucleus is exposed and the oxygen/atom has more electrons than protons. This creates the partial -ve and partial +ve charges making the molecule of water acquire polarity characteristics.
Electronegativity of oxygen cause polarity in water-molecule
The relative electronegativities of the atoms involved is what determines the polarity of bonds determined. These electronegativity values are also determined from a variation of the periodic table of the elements called the periodic table of elements.
Most hydrocarbons (molecules made up of both Carbon and Hydrogen atoms) are extremely nonpolar because of the similarity in the electronegativity of Carbon – Hydrogen. In other words, the hydrogen and carbon atoms share electrons fairly equally.
Polarity caused by electronegativity difference between two atoms in moleculeÂ
Polarity and properties of molecules
Nonpolar molecules do not have significant partial charges, therefore they will not attract polar molecules. Opposite charges attract, similar charges repel one another, but there is no attraction or repulsion between nonpolar molecules. For example, we know that gasoline (a hydrocarbon) will not mix with water. However, if we add oxygen to the hydrocarbon molecules we see that the molecules become polar. Ethanol (drinking alcohol), for instance, completely mixes with water.
Soaps and detergents are unique molecular combinations of polar and nonpolar compounds. Nonpolar part of molecule will react with oils found on soiled hands or clothing while polar part reacts with water. The result is oils removal (nonpolar) from water (high polarity). The polar end of soap is a functional group.
soap made of hydrocarbons and polar end functional group
The incompatibility between nonpolar compounds and water is due to the attraction that water molecules exhibit for one another. Positive charge on the hydrogen of a water-molecule will attract negative charge of atom of oxygen in adjacent water molecule. This attraction, called a hydrogen bond, will cause a type of bond between the molecules themselves.
Because of lack of charges, nonpolar compound won’t have the strength to break the hydrogen bonds between the water molecules. Therefore the nonpolar compound won’t combine with water.
- Published in Water Chemistry, Water Treatment