What is a Covalent Bond
Atoms can bond by sharing electrons in their outermost orbitals and thus giving them the privilege of having a full outermost orbital. For example, the element Hydrogen exists as H2 molecule consisting of two atoms of Hydrogen atom. A covalent bond is simply the force between two atoms resulting from the sharing of electrons in the outermost orbital. Therefore a covalent bond attracts two atoms very close to each others because both atoms share the same electrons in their outermost orbitals.
hydrogen gas formed by covalent bond sharing electron between two hydrogen atoms
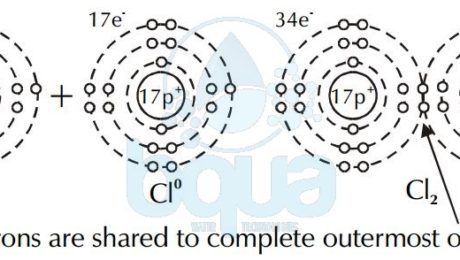
The case of Hydrogen cannot be an ionic bond. That is because Hydrogen ions each have the same charge and therefore are not attracted to one another. This is why a covalent bond happens only when two atoms have a difference in electronegativity of > or = 0.9. Instead, electrons are shared between the atoms to fill the outermost orbital. This type of bond is called a covalent bond. Two atoms of chlorine (Cl0) share a pair of electrons to form chlorine gas (Cl2).
electron shared covalent bond chlorine atom forming chlorine gas
Some molecules formed by covalent bonds may still give up or acquire one or more electrons resulting in a net positive or negative charge. These molecules are called molecular ions.
A covalent bond is very strong. The energy needed to break it is bigger than the thermal energy existing at 25°C which is room temperature. The thermal energy at 25°C is <1 kCal/mole kilo calorie per mole, while to break a typical Carbon covalent bond in an Ethane molecule we need about 83 Kilocalorie per mole.
Properties of molecules defined by covalent bond
While a central atom in a molecule attracts other atoms by covalent bonds, the bonds between these atoms are forming particular angles between them. The force of repulsion among these atoms and particularly the outermost electrons is what shape and determine the angles degrees. The angles and the shape formed is what gives the molecule its properties and shape. An example is the angle formed between Oxygen and Hydrogen atoms in a water molecule, an angle of 105°. You can read about the molecular geometry.
When atoms formed in a covalent bond have the same or very close electronegativity – typically less than 0.9 – the bond (the force of attraction) is equal between electrons since they’re almost identical. This kind of bond is called a non-polar bond. Carbon-Carbon and Carbon-Hydrogen bonds (also called Hydrocarbon) is an example on non-polar bonds.
A polar bond occurs when the difference in electronegativity between two atoms are more than or equal to 0.9. In a polar bond, there’s a partial negative charge (δ−) and a partial positive charge (δ+). This is the case in a water molecule H2O. The bond between Oxygen and Hydrogen is polar since difference in electronegativity is 1.2. (Electronegativity of Oxygen is 3.4 and Electronegativity of Hydrogen is 2.2).
- Published in Water Chemistry, Water Treatment
What is a Crystal Lattice ?
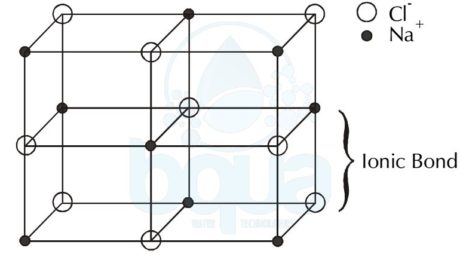
Crystal Lattice is the solid formed by the force of attractions between the charged ions; anions and the cations. It is basically the three dimensional pattern or arrangement of  the atoms inside the crystal.
Since cations are positively charged ions and anions are negatively charged ions, they are strongly attracted to each other. This attraction is very strong that the ions may bond together into a solid called a crystal lattice. The bond that holds the two oppositely-charged ions is called an ionic bond.
Types of Crystal Lattice Structures:
Crystal Lattice exist in seven crystal lattice structures which are:Â
- Cubic
- Monoclinic
- Triclinic
- Hexagonal
- Tetragonal
- Orthorhombic
- Rhombohedral
Each of these crystal lattice structures have unique variants which make the total number of crystal lattice structures 14 Bravais Lattices. The cubic crystal lattice structure has a symmetrical shape and is considered the simplest and most symmetrical form of crystal lattices.
The 3 types of cubic crystal lattice structures are:Â
- Simple Cubic (SC)
- Body-Centered Cubic (CBC)
- Face Centered Cubic (FCC).
Sodium Chloride Crystal Lattice:
An example is the Sodium Chloride – salt – molecule NaCl, since the Sodium (Na) became a cation when it lost an electron to give it to the Chloride atom (Cl) which hence became a negatively charged anion. The ionic bond is the force that is keeping the anions and cations in the solid shape of crystal lattice form.
crystal lattice formed by force of attraction between anions and cations ionic bond
You can also read about Molecular Geometry.
- Published in Water Chemistry, Water Treatment