What is a molecule?
A molecule is a compound that was a substance formed by the chemical bonding of elements. Like an atom is the smallest particle of an element, the smallest discrete particle of a compound is a molecule.
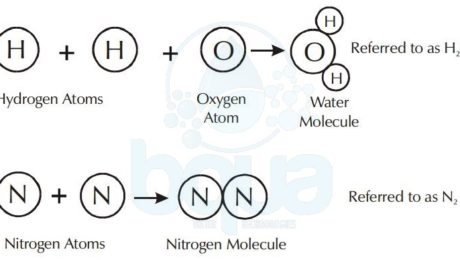
A molecule is made up of atoms from one or more elements. For example, the water molecule consists of two atoms of hydrogen chemically bonded to one oxygen atom. The nitrogen molecule consists of two nitrogen atoms bonded together.
Water Compound formed from Hydrogen Atoms and Oxygen Atoms
One mole equals 6.022×10^23 atoms (602,200,000,000,000,000,000,000 atoms). It is also called Avogadro Number or Avogadro Constant for the man credited for the number. Remember, one mole, or one atomic weight, or one molecular weight, or one Avogadro’s Number of any substance always contains 6.022×10^23 atoms, or molecules of that substance.
In the case of atoms, we describe the weight of one mole; the gram atomic weight. In the case of molecules, we describe it as the gram molecular weight.
Molecules are described by chemical formulas which use the chemical symbols found on the periodic table. Along with numbers to describe the number of atoms of that particular element found in the molecule.
Molecule Name Composition:
– CaSO4 – Gypsum, Calcium Sulfate – One atom of calcium, one sulfur, and four of oxygen.
– NaHCO3 – Baking Soda, Sodium Bicarbonate – One atom of sodium, one atom of hydrogen, one atom of carbon, and three atoms of oxygen.
– Al2(SO4)3 – Alum, Aluminum Sulfate – Two atoms of aluminum, three atoms of sulfur, and twelve oxygen.
– H2S – “Rotten Egg Gas”, Hydrogen Sulfide – Two hydrogens, and one atom of sulfur.
- Published in Water Chemistry, Water Treatment
What is a Functional Group
Molecular compounds owe their individual properties to functional group. These are combinations of atoms which may appear in various parts of the molecule. A list of major functional group listed below.
| Illustrative Example | Name of Functional Class | Functional Group |
| CH3CH2CH3 | Alkanes | C-C |
| CH3CH=CH2 | Alkenes | C=C |
| CH3CH/CH | Alkynes | C/C |
| CH3CH2CH2OH | Alcohols | C–OH |
| Aldehydes | ||
| Ketones | ||
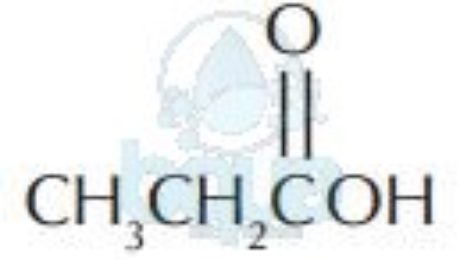
| Acids | ||
| CH3CH2CH2NH2 | Amines | C-NH2 |
| CH3OCH2CH3 | Ethers | -C-O-C- |
| CH3CH2CH2Br | Halides | C-Br |
The difference in electronegativity of two bonding atoms determines if the bond will be polar or nonpolar (read about polarity). The bond between two identical atoms is always nonpolar, (for example: C-C, C=C, C=C; H-H, O=O, etc…). Bonds between atoms with electronegativities which are nearly the same are nonpolar (for example C-H). Bonds between atoms with electronegativities which are significantly different (commonly 0.9) are polar (for example, C-O, H-O, C=O, and N-H).
One molecule, especially a large complex molecule, may have more than one type of functional group. One specific functional group may be repeated several times.
A functional group may alter the properties of a compound such as melting point, boiling point, solid structure, and solubility. A functional group on an organic molecule also contribute to its hydrophobic and hydrophilic properties. Surfaces composed of nonpolar molecules will be hydrophobic. Surfaces composed of compounds containing polar bonds will be hydrophilic. You can also read about surface tension.
- Published in Water Chemistry, Water Treatment
Molecular Weight
A molecular weight is simply the weight of one mole (6.022×10^23 molecules) of a compound. Like the gram atomic weight, in the case of molecules, we describe the weight of one mole (6.022 x 10^23 molecules) as the gram molecular weight. To calculate the gram molecular weight of a molecule, we add the gram atomic weight of each atom comprising the molecule. For example, to calculate the gram molecular weight of water (H2O), we would add the gram atomic weight of oxygen plus two times the gram atomic weight of hydrogen.
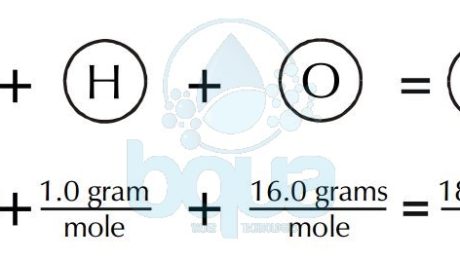
An example of H2O water molecule: The molecular weight is equal to the sum of atomic weights of Hydrogen and Oxygen atoms. Since there are two Hydrogen atoms and one Oxygen. Total is equal to 2(1.0 gram of Hydrogen) + 1(16.0 grams of Oxygen) = 2.0 + 16.0 = 18.0 grams molecular weight. It is also the weight of one mole of water.
Water Compound formed from Hydrogen Atoms and Oxygen Atoms
gram molecular weight of water molecule
Reverse Osmosis membranes will reject ions because of their charge. The membranes will also reject non-charged compounds (like organics) based upon molecular size. Generally, any organic less than 100 molecular weight will not be well rejected. Rejection of organics also depends upon the geometry of the molecule.
Molecular weight is important in organic chemistry since most organic molecules are quite large. Some polymers can have molecular weights in the millions.
- Published in Water Chemistry, Water Treatment