What is Avogadro Number ?
Avogadro Number also known as Avogadro Constant is the number of atoms (6.022 x 1023) in one gram atomic weight (mole) of an element. Or also Avogadro Number can be defined as the number of molecules in a gram molecular weight (mole) of a compound.
It is sometimes given the symbol of NA or L and the unit of measure is mol-1 as per the International System of Units (SI).
Amadeo Avogadro
Avogadro Biography
Full name: Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto, Count of Quaregna and Cerreto. Italian scientist born on the 9th of August 1776 in Turin, Sardinia. Deceased on the 9th of July 1856.
Avogadro 1837 – 1841 published 4 big volumes discussing in details the physics of matter.
Avogadro’s findings and in particular “Avogadro Number” were completely ignored until Stanislao Cannizarro in 1860 presented them at the Karlsruhe Conference. Which was four years after Avogadro left this world. The reason the conference was held is to clarify the confusion that existed at that time about atoms and molecules and their masses.
Still after that Cannizarro presented his findings, not all scientists were convinced. After a decade – with continued strong advocacy from Cannizarro – Avogardo’s hypothesis became more widely accepted and this is when it was called after Avogadro – Avogadro Number.
Today, Avogadro is considered one of the founders of atomic-molecular chemistry
Avogadro Number was first defined and introduced by Jean Baptiste Perrin as the number of atoms in one gram atomic weight of Hydrogen. Which basically means one gram of hydrogen. Later on, Avogadro Number had been redefined as the number of atoms in 12 grams atomic weight of the isotope Carbon-12 (12C). Furthermore, it also relates the amount of a substance to its molecular weight.
| Table shows the value of Avogadro Number  NA in different units |
|---|
| 6.022(74)x1023Â mol-1 |
| 2.731(12)x1026 (lb-mol)-1 |
| 1.7072(77)x1025 (oz-mol)-1 |
- Published in Water Chemistry, Water Treatment
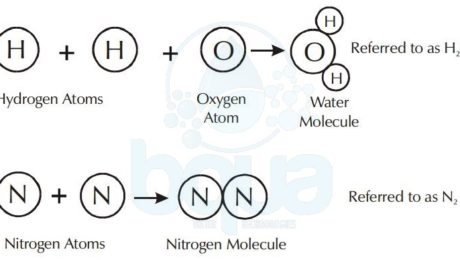
What is a molecule?
A molecule is a compound that was a substance formed by the chemical bonding of elements. Like an atom is the smallest particle of an element, the smallest discrete particle of a compound is a molecule.
A molecule is made up of atoms from one or more elements. For example, the water molecule consists of two atoms of hydrogen chemically bonded to one oxygen atom. The nitrogen molecule consists of two nitrogen atoms bonded together.
Water Compound formed from Hydrogen Atoms and Oxygen Atoms
One mole equals 6.022×10^23 atoms (602,200,000,000,000,000,000,000 atoms). It is also called Avogadro Number or Avogadro Constant for the man credited for the number. Remember, one mole, or one atomic weight, or one molecular weight, or one Avogadro’s Number of any substance always contains 6.022×10^23 atoms, or molecules of that substance.
In the case of atoms, we describe the weight of one mole; the gram atomic weight. In the case of molecules, we describe it as the gram molecular weight.
Molecules are described by chemical formulas which use the chemical symbols found on the periodic table. Along with numbers to describe the number of atoms of that particular element found in the molecule.
Molecule Name Composition:
– CaSO4 – Gypsum, Calcium Sulfate – One atom of calcium, one sulfur, and four of oxygen.
– NaHCO3 – Baking Soda, Sodium Bicarbonate – One atom of sodium, one atom of hydrogen, one atom of carbon, and three atoms of oxygen.
– Al2(SO4)3 – Alum, Aluminum Sulfate – Two atoms of aluminum, three atoms of sulfur, and twelve oxygen.
– H2S – “Rotten Egg Gas”, Hydrogen Sulfide – Two hydrogens, and one atom of sulfur.
- Published in Water Chemistry, Water Treatment