What is Forward Osmosis
Fundamentally, Forward Osmosis exploits a naturally occurring phenomenon (Osmosis). Which is simply the water transport over a semi-permeable membrane from a low concentration to a high concentration. In a perfect world, the semi-permeable membrane permits water to pass through it however rejects all salts and undesirable components. The high salinity solution acts as the draw solution, which has a higher concentration than the feed water. To initiate the passage and attract water to pass through the membrane from the feed side to itself. In this manner, Forward Osmosis requires less energy (applied pressure) to transport a water stream through the membrane. In comparison to the pressure driven membrane procedures, for example, reverse osmosis (RO). However, as opposed to Reverse Osmosis, the result of Forward Osmosis is not fresh water but rather a diluted draw solution. It is a blend of the feed water and draw solution. In this way, a following filtration or separation process must be performed to extract clean water and to recover the draw solution. The draw solution consists of either a single or multiple simple salts or can be a substance specifically tailored for forward osmosis applications.
The following step of filtration might be energy intensive relying upon the draw solution and the recycling procedure. Hence, for potable water production, one must consider the energy utilization of both the Forward Osmosis procedure and the DS recovery. So that we can eventually make a reasonable examination and run comps between Forward Osmosis and other water treatment advancements. Or else, the conclusion could be one-sided and deceiving. In any case, Forward Osmosis might be more practical than pressure driven membrane technologies for water reuse. Only if the recovery of the Draw Solution is not required. Consequently, Researches and developments made on Forward Osmosis will be used to organize those procedures and applications without reusing the Draw Solution.
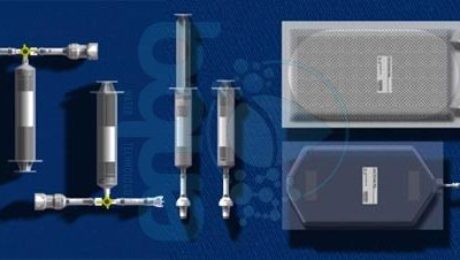
One of the most significant applications for Forward Osmosis is done by NASA. Six forward osmosis kits will fly aboard space shuttle Atlantis on the STS-135 mission by NASA. Scientists from NASA’s Kennedy Space Center in Florida plan to test a space adapted version of a Forward Osmosis bag. This will happen aboard space shuttle Atlantis during the STS-135 mission summer 2017. The group at Kennedy, led by NASA Project Manager Spencer Woodward, will include in the shuttle’s cargo six forward osmosis bag kits for the astronauts to test. The bags’ manufacturer, made a few adaptations to their commercial product for spaceflight. The idea is to make a fortified drink that provides hydration and nutrients from all sources available aboard a spacecraft, such as wastewater and even urine.
Forward Osmosis bag kit NASA experiment
Up until today, Forward Osmosis still experiences issues as a cost-effective innovation for direct seawater desalination. As a result of its high energy utilization and absence of efficient DS with -close to – negligible reverse flux. Regardless of many innovative advances in DS recently made, challenges still exist to: First, limit the reverse flux of DS. Secondly, mitigate internal concentration polarization (ICP) and Lastly, find better and easier recovery strategies. In conclusion, Forward Osmosis represents a forthcoming technology with a very low impact on the environment.
- Published in Technology, Water Treatment
What is Osmotic Pressure
Osmotic pressure is a function of dissolved substances. As in Osmosis, water will pass through a semi-permeable membrane from the lower concentration compartment into the higher concentration compartment. Osmotic pressure is simply what makes osmosis process occurs. The force of this flow is measured by measuring how much pressure must be applied to the higher salt concentration side in order to stop osmosis. This pressure then must be the force of osmosis. This pressure is called osmotic pressure. Osmotic pressure is a function of the number of collisions of water molecules with either side of the membrane. The more dissolved substances, of any kind, there are, the fewer the water collisions.
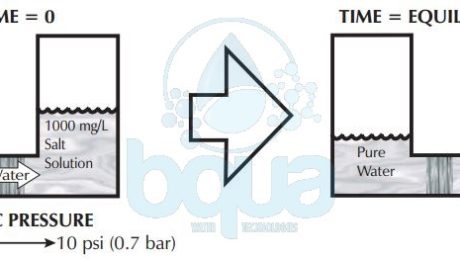
A very rough rule of thumb is that for every 100 mg/L or ppm of TDS (Total Dissolved Solids) the osmotic pressure is roughly 1 psi (0.07 bar). If pure water is separated from a 1000 mg/L TDS solution by a semi-permeable membrane. It will take around 10 psi of pressure on the 1000 mg/L side in order to stop osmosis. Therefore, the osmotic pressure forcing water from the pure water side into the 1000 mg/L side is 10 psi. While the greater water collisions are from the pure water side of the membrane, the reason for water movement left to right is due to the higher salt concentration on the right hand side.Â
osmotic pressure is what makes osmosis process occurs
Amount of osmotic pressure generated, therefore, is directly proportional to the amount of total dissolved solids (TDS) in the solution. Since every 100 mg/L creates around 1 psi (0.07 bar) of osmotic pressure, we simply have to divide the TDS by 100 (move the decimal to the left two places) in order to calculate the approximate pressure.
Now what happens when we have salt solutions on both sides of a semi-permeable membrane? Net osmotic pressure becomes important. The net pressure is the difference between the pressure of each of the solutions separated by a semi-permeable membrane. If two 1000 mg/L salt solutions separated by semi-permeable membrane, roughly 10 psi of osmotic pressure is being exerted by each solution. In this case the pressures are equal and opposite in direction. Substracting one from the other, the net osmotic pressure is zero. This is when the osmosis process reaches equilibrium.
- Published in Technology, Water Treatment
What is Osmosis Definition
Osmosis is a natural process (phenomenon) that occurs in nature around us. The Osmosis process is even happening now within our bodies while reading this post.
A simple definition of Osmosis is that it is the tendency of a fluid to pass or flow through a semipermeable membrane into solution of higher concentration. Osmosis is the process whereby the water that we drink eventually ends up in our blood, muscles, and cells. The lining in our intestine is a membrane. The yolk of an egg has a membrane surrounding it that releases its contents when broken. If we simply wrap a piece of meat with plastic wrap, we can consider the plastic wrap a membrane.
A definition of permeable is when materials can pass through it. For example, a kitchen strainer or sieve is totally permeable to water. Meaning that water passes freely through it. Semipermeable means that some materials pass while others don’t. The kitchen strainer is an example. Since it allows water to pass freely through it but will not allow large solids to infiltrate.
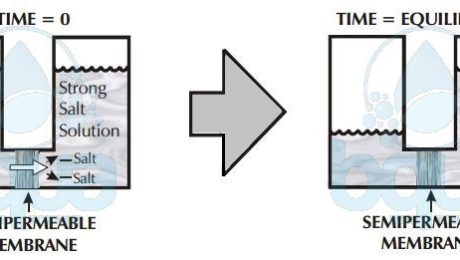
Back to Osmosis, water passes through semi-membrane from low salt concentration into a solution of high concentration. The reason is simple, let’s imagine having a salt solution and pure water separated by such a membrane. We know that water molecules may freely pass through the membrane but dissolved substances can’t. Given that all molecules are in motion, which liquid will have the greatest number of molecules colliding with the membrane? The pure water or the salt water?
Per given area of membrane, the pure clean water side will have more molecules of water that collide with the membrane than the side of water with salt. Water in Osmosis, therefore will pass from the side with pure or clean water to the salt water side. The higher the concentration of salt, the fewer the collisions of water molecules with the membrane. In Osmosis, water always passes through a semi-permeable membrane, therefore, from a lower salt concentrated side into a higher concentrated side.
osmosis: lower salt concentration to higher concentration
Osmosis then describes the tendency of fluid (water) to pass through a semi-permeable membrane, into a solution of higher concentration. The process stops when the number of water molecules colliding with either side of the membrane are equal. The number of collisions is a function of the salt concentration and the pressure on whichever membrane side. These are called osmotic pressure and applied pressure.
Osmosis reaches equilibrium when the concentration of dissolved solids at both compartments is equal. Meaning no more net flow from one side to another. The side that was the higher concentration solution however now has a higher water level than the other side due to osmosis. This difference in height between both sides is due to osmotic pressure.
Now that you learned about Osmosis, you might want to read about Reverse Osmosis (RO).
- Published in Water Chemistry, Water Treatment