Posts under this category displays latest news, useful articles, terms definitions, scientific explanations and more topics related to the water treatment industry.
What is Osmosis Definition
Osmosis is a natural process (phenomenon) that occurs in nature around us. The Osmosis process is even happening now within our bodies while reading this post.
A simple definition of Osmosis is that it is the tendency of a fluid to pass or flow through a semipermeable membrane into solution of higher concentration. Osmosis is the process whereby the water that we drink eventually ends up in our blood, muscles, and cells. The lining in our intestine is a membrane. The yolk of an egg has a membrane surrounding it that releases its contents when broken. If we simply wrap a piece of meat with plastic wrap, we can consider the plastic wrap a membrane.
A definition of permeable is when materials can pass through it. For example, a kitchen strainer or sieve is totally permeable to water. Meaning that water passes freely through it. Semipermeable means that some materials pass while others don’t. The kitchen strainer is an example. Since it allows water to pass freely through it but will not allow large solids to infiltrate.
Back to Osmosis, water passes through semi-membrane from low salt concentration into a solution of high concentration. The reason is simple, let’s imagine having a salt solution and pure water separated by such a membrane. We know that water molecules may freely pass through the membrane but dissolved substances can’t. Given that all molecules are in motion, which liquid will have the greatest number of molecules colliding with the membrane? The pure water or the salt water?
Per given area of membrane, the pure clean water side will have more molecules of water that collide with the membrane than the side of water with salt. Water in Osmosis, therefore will pass from the side with pure or clean water to the salt water side. The higher the concentration of salt, the fewer the collisions of water molecules with the membrane. In Osmosis, water always passes through a semi-permeable membrane, therefore, from a lower salt concentrated side into a higher concentrated side.
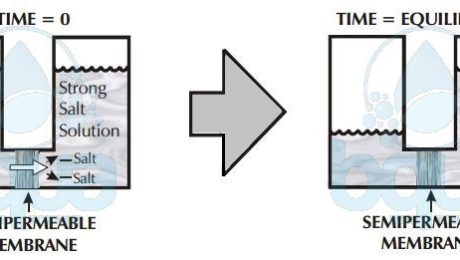
osmosis: lower salt concentration to higher concentration
Osmosis then describes the tendency of fluid (water) to pass through a semi-permeable membrane, into a solution of higher concentration. The process stops when the number of water molecules colliding with either side of the membrane are equal. The number of collisions is a function of the salt concentration and the pressure on whichever membrane side. These are called osmotic pressure and applied pressure.
Osmosis reaches equilibrium when the concentration of dissolved solids at both compartments is equal. Meaning no more net flow from one side to another. The side that was the higher concentration solution however now has a higher water level than the other side due to osmosis. This difference in height between both sides is due to osmotic pressure.
Now that you learned about Osmosis, you might want to read about Reverse Osmosis (RO).
- Published in Water Chemistry, Water Treatment
Radium Removal
Radium 226 and radium 228 are natural groundwater contaminants that usually occur at trace levels. For any water treatment application, radium removal is substancial since its presence cause serious health problems and even death at some point. Radium A strong acid cation exchange resin operated in the sodium cycle is a very effective method of radium removal.
Radium is like barium, it has a higher selectivity for cation exchange resins than hardness. It will also be removed during the normal water softening cycle. In a manner similar to barium, on the first cycle of exhaustion, radium will continue to load on the resin bed until well after hardness breakthrough by displacing all the other ions previously loaded, including calcium and magnesium. This effect is valid only for the first cycle and can be misleading.
Radium removal water treatment radium 226 radium 228
Point-of-use cartridges, which use ordinary softener resins on a one-time basis for radium removal. Will provide decent radium removal for over 5 to 15 times. This depends on the particular resin as long as they will produce softened water. In systems that can be regenerated, however, radium like barium. This means it is much harder to regenerate off the resin and more of it will remain in the resin after regeneration. The radium will be pushed toward the exit (bottom) of the resin bed during the regeneration cycle. As the softener becomes exhausted, hardness leakage reaches the radium-rich end of the resin bed.
The hardness, which is less preferred by the resin, will displace only a small, but nevertheless, significant amount of radium from the resin, causing radium levels to increase to unacceptable levels. Therefore, in systems that are regenerated it is necessary to limit the service cycle to the softener capacity for hardness. Since the amount of radium is insignificant compared to hardness, the softener design calculations are made for an ordinary softener.
Learn more about BQUA’s water treatment products
- Published in Water Treatment
Boiler Feed Water Properties
Requirements for boiler feed water design and requirements include maximum tolerance levels of alkali, salt, silica, phosphates and other elements. This is in proportion to the pressure at which the boiler will be operated. These boiler water levels must be gotten from the boiler manufacturer, and are basically based on the boiler water properties.
water treatment scheme for boiler feed water
Below tables are the recommended boiler feed water properties / boiler feed water chemical characteristics (levels) Â taken from APAVE. Which is the Association of electrical and steam unit owners), up to pressures of 100 bar for medium steaming rates and for volumes of water in the chambers sufficient to properly control the blow down rates, and from ABMA (American Boiler Manufacturers Association) in its standard guarantee of steam purity.
| Boiler Working Pressure (Bar) | |||||||||
| 0 – 20.7 | 20.8 – 31.0 | 31.1 – 41.4 | 41.5 – 51.7 | 51.8 – 62.1 | 62.2 – 68.9 | 69.0 – 103.4 | 103.5 – 137.9 | ||
| Boiler Feed water | |||||||||
| Dissolved oxygen (measured before oxygen scavenger addition) | 0.04 | 0.04 | 0.0070 | 0.007 | 0.0070 | 0.007 | 0.007 | 0.007 | |
| Total Iron | mg/l | 0.1 | 0.05 | 0.03 | 0.025 | 0.02 | 0.02 | 0.01 | 0.01 |
| Total copper | 0.05 | 0.025 | 0.02 | 0.02 | 0.015 | 0.015 | 0.01 | 0.01 | |
| Total hardness(CaCO3) | 0.3 | 0.3 | 0.20 | 0.2 | 0.1 | 0.05 | not detectable | ||
| Non volatile TOC | 1 | 1.0 | 0.5 | 0.5 | 0.5 | 0.20 | 0.2 | 0.2 | |
| Oily matter | 1.0 | 1 | 0.50 | 0.5 | 0.50 | 0.2 | 0.2 | 0.2 | |
| pHÂ at 25 | 7.5 – 10 | 7.5 – 10 | 7.50 – 10.0 | 7.50 – 10.0 | 7.50 – 10 | 8.5 – 9.5 | 9.0 – 9.6 | 9.0 – 9.6 | |
| Boiler Water | |||||||||
| Silica | mg/l | 150 | 90 | 40 | 30 | 20 | 8 | 2 | 1 |
| Total alkalinity (CaCO3) | 350 | 300 | 250 | 200 | 150 | 100 | not specified | ||
| Free hydroxide alkalinity CaCO3 | not specified | not detectable | |||||||
| Specific conductance at 25 without neutralization | mS/cm | 3500 | 3000 | 2500 | 2000 | 1500 | 1000 | 150 | 100 |
| Boiler Working Pressure (Bar) | ||||||||
| 0 – 15 | 15 – 25 | 25 – 35 | 35 – 45 | 40 – 60 | 60 – 75 | 75 – 100 | ||
| Boiler Feed water | ||||||||
| Dissolved oxygen (measured before oxygen scavenger addition) | mg/l | 0.02 (Physical removal of dissolved oxygen) | ||||||
| Total hardness | French degrees | 0.5 | 0.3 | 0.2 | 0.1 | 0.05 | 0.05 | 0.05 |
| Oily matter | mg/l | absence | 0.05 | 0.05 | 0.05 | |||
| pH | > 8.5 | |||||||
| Total Iron | mg/l | not specified | 0.05 | 0.05 | 0.03 | |||
| Total copper | not specified | 0.03 | 0.03 | 0.01 | ||||
| Boiler water | ||||||||
| MÂ alkalinity | French degrees | 100 | 80 | 60 | 40 | 15 | 10 | 5 |
| P alkalinity | 0.07 M | 0.070 M | 0.07 M | 0.070 M | > 0.5 M | > 0.5 M | > 0.5 M | |
| SiO2 | mg/l | 200 | 150 | 90 | 40 | 15 | 10 | 5 |
| TDS | 4000 | 3000 | 2000 | 1500 | 500 | 300 | 100 | |
| Phosphates | 30 to 100 | 31 to 100 | 20 to 80 | 21 to 80 | 10 to 60 | 10 to 40 | 5 to 20 | |
| pH | 10.5 to 12 | 10 to 11 | ||||||
| Make up water | Softened or softened and carbonate free | Demineralized | ||||||
- Published in Water Treatment
What is a Crystal Lattice ?
Crystal Lattice is the solid formed by the force of attractions between the charged ions; anions and the cations. It is basically the three dimensional pattern or arrangement of  the atoms inside the crystal.
Since cations are positively charged ions and anions are negatively charged ions, they are strongly attracted to each other. This attraction is very strong that the ions may bond together into a solid called a crystal lattice. The bond that holds the two oppositely-charged ions is called an ionic bond.
Types of Crystal Lattice Structures:
Crystal Lattice exist in seven crystal lattice structures which are:Â
- Cubic
- Monoclinic
- Triclinic
- Hexagonal
- Tetragonal
- Orthorhombic
- Rhombohedral
Each of these crystal lattice structures have unique variants which make the total number of crystal lattice structures 14 Bravais Lattices. The cubic crystal lattice structure has a symmetrical shape and is considered the simplest and most symmetrical form of crystal lattices.
The 3 types of cubic crystal lattice structures are:Â
- Simple Cubic (SC)
- Body-Centered Cubic (CBC)
- Face Centered Cubic (FCC).
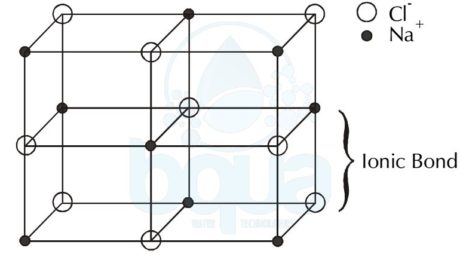
Sodium Chloride Crystal Lattice:
An example is the Sodium Chloride – salt – molecule NaCl, since the Sodium (Na) became a cation when it lost an electron to give it to the Chloride atom (Cl) which hence became a negatively charged anion. The ionic bond is the force that is keeping the anions and cations in the solid shape of crystal lattice form.
crystal lattice formed by force of attraction between anions and cations ionic bond
You can also read about Molecular Geometry.
- Published in Water Chemistry, Water Treatment
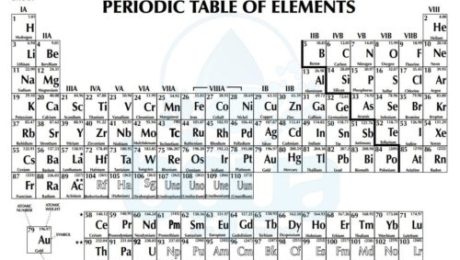
What is Periodic Table of Elements:
Atoms of different elements are distinguished only by the number of subatomic particles; electrons, protons and neutrons. Scientists decided to organize the elements in a periodic table based on the number of these particles in the atoms of each element. By the time, a chart was developed which described the atom structure of each element. This chart has come to be known as the periodic table of elements.
periodic table of elements including atomic number atomic weight
By observing the periodic table there are a lot of important information for each element. We see that each element in the periodic table has a Chemical Symbol which is usually the first one or two letters of the element’s name. For instance: H for Hydrogen, He for Helium, O for Oxygen, Ca for calcium, etc. In other cases, there are elements that have symbols which do not correspond to the name of the element. For instance: Na for sodium, K for potassium, etc. These letters are abbreviations of the Latin name of these elements. Na are the first two letters of Natrium (Sodium in Latin), K is the first letter of Kalium (Potassium in Latin) and so on.
Another observation in the periodic table is that each element has a corresponding number. This number is called the atomic number and it refers to the number of protons in the nucleus. Since we know that in neutral atoms (not charged) the number of protons is equal to the number of electrons, the atomic number also equals the number of electrons.
atomic number and atomic weight in periodic table of elements
The number which is greater than the atomic number is called the atomic weight. The atomic weight also called gram atomic weight is the weight of a particular number of atoms of the element in grams.
- Published in Water Chemistry, Water Treatment
Boiler Water Treatment
Boiler water treatment is one type of industrial water treatment which is focused on removing both organic and inorganic elements that can cause damages to the boiler. Water treatment takes places to avoid scaling, corrosion, or foaming. Raw or feed boiler water is treated before the contaminants/impurities reach the boiler. Internal boiler water treatment’s purpose is to prevent dissolving of the boiler and to keep contaminants in certain formations and levels that do not harm it or cause a blow down.
Water can be easily and quickly heated when exposed to a temperature rise than any other inorganic matter. When it evaporates and turns into steam at atmospheric pressure it dilates by one thousand and six hundred times. While in form of steam, it is charged with a huge amount of heat, and with these special characteristics water has it makes a special element for boilers, heaters and power generators.
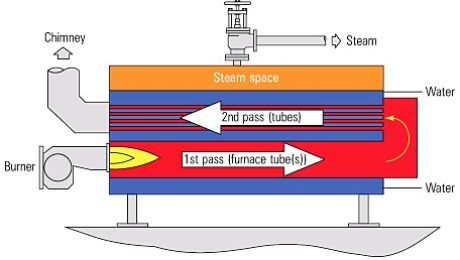
There are basically many types of boilers, among them are only two types of boilers that are mostly and widely used today. There is the fire tube boiler and the water tube boiler. Both have different yet opposite mechanism of operation. The fire tube boiler has its heat source located inside a furnace or a fire box that is surrounded by water. This is in order to maintain the temperature of the heating surface below the boiling point.
As in case water tube boilers, it is completely the opposite. Water and steam flow inside the tubes and the hot gases flow over the outside surface. The circulation system in the water tube boiler is constructed of tubes, headers, and drums joined in arrangement that provide water flow to generate steam.
Boiler Make up Water
Water contains both suspended and dissolved solids and gases in assorted quantities. Varying from 30 grams/liter for seawater and ranging from 0.005 to 1500 milligrams/liter for fresh water. These water contaminants cause erosion and corrosion for boilers and yet other issues. This is when water treatment is considered for boiler make up water in order to bring out a certain standard and quality. Purpose is to make boiler water safe to be used in generation of steam in steam generators and boilers.
Every boiler has a design that requires a certain level of impurities. And this level cannot be exceeded in the water being used. It is more like a tolerance that is limited and if exceeded it can cause serious deterioration of the boiler. This is why boiler water with high amounts of impurities has to be pretreated and contaminants has to completely removed before being transformed into steam to improve quality.
Both quantity of nature of impurities in the boiler water are considerable. For instance hardness, iron and silica are of high concern than sodium salt. Flow rate and design of the boiler are highly considered factors in treatment of feed boiler water. Also of these factors; pressure, heat transfer rate, etc. A low pressure fire tube boiler can tolerate high level of hardness with the use of a certain treatment. Contaminants must be completely be removed in case of modern boilers that use high pressure.
BQUA is proudly capable of supplying you with the necessary and suitable equipment in order to treat your boiler feed water prior to entering the equipment. This to make sure no scaling or fouling would occur inside of the boiler and guarantee a long service life without any problems. Please contact our technical sales engineers for more information.
Read more about the boiler water characteristics and feed make-up water properties
- Published in Water Treatment
What is Process Water ?
Process water is the name given for water which is not considered drinkable (not drinking water) and is basically used in relation to industrial plants, industrial processes and production facilities. Process water was originally subjected to a substantial water treatment, like for instance; multimedia filters, softeners, reverse osmosis, etc.
Process Water in the industrial water treatment process
Most probably each and every product being manufactured uses industrial water treatment during some if not all stages of the production procedure. Industrial water treatment main purpose is to facilitate the manufacturing process, reduce cost and help support production for certain products of which water is an essential element in its composition; i.e: beverages processing, detergents, paper, chemical processing, etc. The use of process water can be in many forms such as; Â fabrication, dilution, sanitation or incorporation of process water into a product.
It should typically have conductivity 0.1 to 50 Microsiemens per Centimeter µS/cm, with almost zero hardness to to prevent scaling. Carbon dioxide (CO2) and Oxides (Oxygen) cause corrosion. Water requirements depends on the type of application.
Process Water in the commercial water treatment process
Commercial water treatment sector is essential  when thinking of the use of process water in steakhouses, every restaurant and cafe, pizzerias, hotels, offices, shops, civil and military organizations and all other sorts of commercial water uses.
With the required knowledge and expertise and a broad product range, BQUAÂ is proudly capable to implement and accomplish projects for all types of applications and harsh solutions.
- Published in Water Treatment
Water Treatment Oil and Gas
Purpose of Reverse Osmosis Systems is to support and help treatment of oil contaminated water to recover petroleum oils that are found in water bodies at several stations.
- Free non-emulsified oils
- Emulsified oils and stable in the water
- Insoluble solids
The principles of the separation, recovery and water treatment oil and gas depends on characteristics and properties of these oils; the nature and existence, solid materials and non-emulsified oils are physically separated while emulsified oils are chemically separated. Finally, small emulsions and organic dissolved material which were not treated with previous means are being treated biologically.
Stages and methods for water treatment oil and gas
Contaminated water are being processed by three phases depending on the method of processing which are:
- Physical treatment
- Chemical processing treatment
- Biological treatment stage
Physical treatment
Solid matters and non-emulsified oils are separated based on the principle of gravity, where design breaks is returned to the American Petroleum Institute, which the design depends on the principle of the difference in density between the water and oils properties, whether free or solid. Free oils float on the surface of the water and taken by a certain abrasive raze to a special hole to be returned again after layering to the production lines while the solid matters settle at the bottom of these basins are taken through a private in the form of sludge sockets to private places where follow up treatment and disposal.
Chemical processing treatment
Emulsified oils can not be separated physically so it needs to be separated chemically which allows to remove the case of emulsification and stability emerging between oil and water in the middle surrounding the second phase.
Where external water are brought from external basins after adding some coagulated materials such as iron sulfate which is less expensive than other coagulated materials and because they in fact do two jobs.
Forms (after oxidation by dissolved oxygen moiety) hydroxide compound of iron gelatin textures and surface, which has a capacity that helps adsorption of emulsified oil droplets on the surface and the air with the help of the dissolved.
The addition of iron sulfate helps in getting rid of hydrogen sulfide gas dissolved and compounds which may be contained with oily water, which have seriously damaged the subsequent biological treatment work, where the presence of iron sulfide is a moiety with ion iron deposit is a black iron sulfide.
After the formation of the raw material adsorption (ferric hydroxide) a polymer is added with high molecular weights that carries an electric positive charge on the surface collects iron hydroxide particles on the surface compounds the problem so volumes and surfaces wide capable of dissolved air flotation, which will inject later assisted.
Complete chemical treatment of so-called phase flotation stage, a very important and very critical stage in the improvement of the final specifications for water treatment and symbolized by the acronym of the dissolved air flotation.
The principle of this method depends on the density change problem solids by adding the previous chemicals by joining the air bubbles injected by a private network at the bottom of the tub to the surfaces of these pendants and its contribution to the expansion of the relative surfaces and thus reduce the intensity of which will allow its flotation.
In other words, what is meant by flotation in fact is the updraft of adsorption materials that will contribute to the adsorption of emulsified oil droplets at the same time also pick up solid natural pendants which we call water turbidity collected on the surface of the flotation tanks.
Then to be taken by a special raze gathered in private protectors called flotation sludge pit.
Biological treatment stage
Emerging from the flotation stage water enters the biological treatment providers basins mechanical mixers are secure ventilation of these basins and providing oxygen required for processes of oxidation where this method is the most common and successful way in converting organic material, whether decadent or sizes minutes impossible separated previous stages to non-material decadent through oxidized by microorganisms (bacteria) that are converted via vital metabolism to carbon dioxide and to new microorganisms called bacterial midwife to be placed at the bottom attached to the biological reactors settling basins and described in the third stage in the scheme.
Many of the microorganisms can feed on organic material dissolved or suspended and disassembled condition of maintaining the appropriate conditions of her life and, in particular, needs oxygen.
And measured the water content of organic material degradable bacteria, including the requirement we call organic oxygen: BOD Biochemical Oxygen Demand.
This standard represents the amount of oxygen consumed by bacteria to represent this organic material and can be called organic pregnancy basin biological When this pregnancy is fairly low and if there is sufficient tracts of land are designed in this case the biological basins Allagoonat system or open pits where believes bacteria which need oxygen directly from the natural air.
But if the high organic load must in this case from those basins oxygen supply mechanical means which are periodically required to secure for the entire basin ventilation.
- Published in Water Treatment
Welcome to BQUA’s Water Treatment Blog
We are going to share the latest information and news regarding the top of the line applied water treatment technologies and many other water related topics. Posts will also focus on commercial and industrial Reverse Osmosis, seawater desalination, water treatment solutions, and other various water purification systems and applications.
Keep an eye on our Reverse Osmosis Water Treatment Blog to keep yourself and your company updated with the latest applied water technologies in the science of purification and treatment of water.
- Published in Water Treatment