Posts under this category displays latest news, useful articles, terms definitions, scientific explanations and more topics related to the water treatment industry.
Molecular Weight
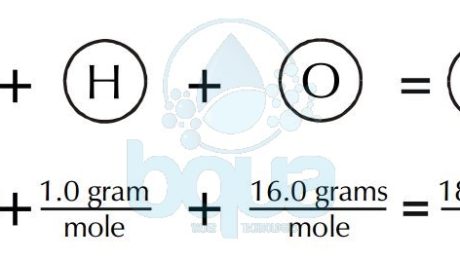
A molecular weight is simply the weight of one mole (6.022×10^23 molecules) of a compound. Like the gram atomic weight, in the case of molecules, we describe the weight of one mole (6.022 x 10^23 molecules) as the gram molecular weight. To calculate the gram molecular weight of a molecule, we add the gram atomic weight of each atom comprising the molecule. For example, to calculate the gram molecular weight of water (H2O), we would add the gram atomic weight of oxygen plus two times the gram atomic weight of hydrogen.
An example of H2O water molecule: The molecular weight is equal to the sum of atomic weights of Hydrogen and Oxygen atoms. Since there are two Hydrogen atoms and one Oxygen. Total is equal to 2(1.0 gram of Hydrogen) + 1(16.0 grams of Oxygen) = 2.0 + 16.0 = 18.0 grams molecular weight. It is also the weight of one mole of water.
Water Compound formed from Hydrogen Atoms and Oxygen Atoms
gram molecular weight of water molecule
Reverse Osmosis membranes will reject ions because of their charge. The membranes will also reject non-charged compounds (like organics) based upon molecular size. Generally, any organic less than 100 molecular weight will not be well rejected. Rejection of organics also depends upon the geometry of the molecule.
Molecular weight is important in organic chemistry since most organic molecules are quite large. Some polymers can have molecular weights in the millions.
- Published in Water Chemistry, Water Treatment
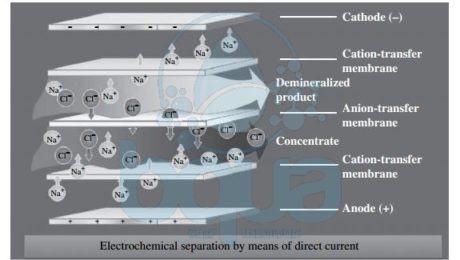
What is Continuous Electrodeionization (CEDI)
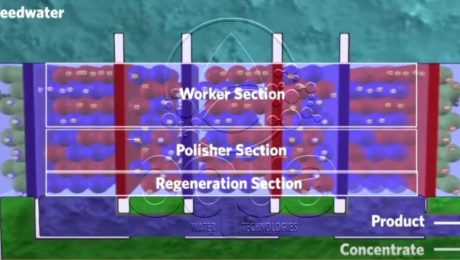
The continuous electrodeionization is a recent invention that was brought by a water treatment company which patented the CEDI technology. CEDI which is the abbreviation for the continuous electrodeionzation is considered as a smart evolution to conventional Electrodialysis Reversal (EDR) technology. It is introduced as a blend of ion exchange membranes, ion exchange resins and electricity. The difference between the resins used in this technology and the conventional Ion Exchange resins made of divinyl benzene (DVB) is that these resins are continuously regenerated in the continous electrodeionzation without the need for regeneration chemicals or salt.
The way Continuous Electrodeionization CEDI works is so simple. It is actually very similar to Electrocoagulation in respect to the use of plates/electrodes (anode and cathode). Applying a DC current to plates, it turns them to anode and cathode. This is done in order to attract dissolved solids consisted of mainly anions (negatively charges ions) and cations (positively charged ions). The anode electrode will attract negatively charged ions while the cathode will attract positively charged ions. When ion exchange membranes made of cation selective resins are inserted right close to the cathode, it will block the passage of anions and water molecules. On the other hand, when we insert an ion exchange membrane made of anion selective resin close to the anode, it will block the passage of cations and water molecules and only allow anions to pass.
Continuous Electrodeionization Operation and Resin Regeneration
This configuration of membranes and electrodes form the framework of a Continuous Electrodeionization CEDI module. This process is however slowed down by the slow speed of which ions move in water, in fact the low conductivity of water molecules impedes ions removal. Meaning, as ions move outward, the water in the dilute chamber become purified. As ion levels decreases, electrical resistance increases and eventually the whole process slows down. That was solved by adding anions and cations selective resin beads between the two Ion Exchange membranes which reduces the electrical resistance.
The surface of the beads in the continuous electrodeionization CEDI module acts as an ion transport bridge. So that the ions can move quicker through the membranes at to the electrodes. Continuously adding resin beads – Ion Exchange selective membranes sandwiches, creates a series of water purification compartments where product and brine exit the system. As feed water is pumped into the system, it is diverted into separate compartments: concentrate and purification compartments. These two streams remain separated throughout the process because only ions can pass through the membranes. Ions migrate and accumulate in the concentrating compartment where they are washed away into the reject stream; exit the system as concentrate. The water leaving this compartment contain a concentration of ions of approximately 10 – 20x higher than the original feed water. This water can be either drained, recycled or reclaimed for further treatment.
Continuous Electrodeionization CEDI module
At the top of the purification compartment the ion concentration is at its highest. The surface of the resin beads act as a conductive path effectively moving the ions to the membranes. At the lower end of the purification, the ion concentration is reduced to the parts per trillion (ppt) level. The electric field becomes concentrated between the resin beads and the surrounding water resulting an electrochemical reaction. Where water splitting occurring into Hydrogen and Hydroxide ions which is essentially acid and caustic. The acid and caustic generated is what regenerated the resin beads by replacing other trace ions remaining. This exactly what happens in conventional IX deionization systems. A result is a chemical free operation where the electrical potential does all the work and extends the life of the resin.
- Published in Technology, Water Treatment
What is a Pressure Membrane / Pressure-Driven Membrane
The pressure membrane processes are:
– Reverse osmosis (RO)
– Nanofiltration (NF)
– Ultrafiltration (UF)
– Microfiltration (MF)
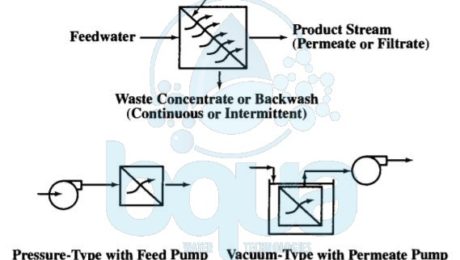
A pressure membrane is a membrane that functions by applying a pressure. A pressure membrane is permeable to water but not to substances which are rejected and removed. All membranes including any pressure-driven membrane separate feedwater into two streams: permeate and concentrate streams. The permeate (for RO, NF, or UF) or filtrate (for MF) stream passes through the membrane barrier. The concentrate (or retentate) stream contains the substances removed from the feedwater after the pressure membrane barrier rejects it.
Actually, the driving force for these pressure membrane processes may come from (1) a pressurized feedwater source with the membranes installed in pressure vessels, called modules. Or (2) a partial vacuum in the filtrate/permeate flow stream caused by use of a filtrate/permeate pump or gravity siphon. The vacuum-driven processes typically apply to MF and UF only and have membranes submerged or immersed in nonpressurized feedwater tanks.
pressure membrane process using feed or permeate pumps
Pressure membrane processes are designed for cross-flow or dead-end operating modes. In the cross-flow mode, the feed stream flows across the pressure membrane surface. And permeate (or filtrate) passes through the pressure-driven membrane tangential to the membrane surface. Moreover, cross-flow operation results in a continuously flowing waste stream. A cross-flow system design sometimes contain a concentrate recycle. Also with a reject stream (feed-and-bleed mode). Many MF and UF systems treating relatively low turbidity waters are also designed to operate in a dead-end flow pattern where the waste concentrate stream is produced by an intermittent backwash. The figure below shows the relative removal capabilities for pressure-driven membrane processes and compares these processes with media filtration.
pressure membrane processes RO NF UF MF difference pore size rejection
In fact, MF and UF separate substances from feedwater through a sieving action. Separation depends on the pressure membrane pore size and interaction with previously rejected material on the membrane surface. Furthermore, NF and RO separate solutes by diffusion through a thin, dense, permselective (or semi-permeable) membrane barrier layer, as well as by sieving action. The required pressure membrane feed pressure generally increases as removal capability increases.
- Published in Technology, Water Treatment
What is Surface Tension
Surface tension is one of the properties of water that is created by the Hydrogen bond. Surface tension is the reason a water droplet can have different shapes on different surfaces. As we all noticed, when we fill a glass of water to the top, water level may actually be above the glass rim. The explanation for this occurrence is the high surface tension of water on a hydrophilic surface like glass due to hydrogen bond.
surface tension of water on plastic hydrophobic
surface tension of water droplet on hydrophilic glass
Hydrophobic and Hydrophilic Examples
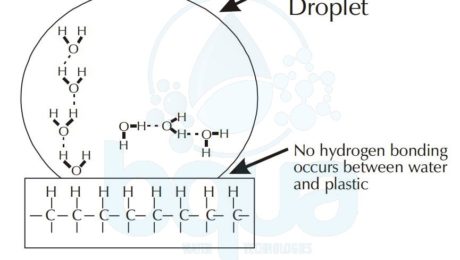
On the other hand, a water droplet tends to form a sphere like shape on a hydrophobic surface like for example plastic. Therefore, a drop of water will tend to spread on a clean glass surface. To simply explain why this happens, we will analyse the phenomenon on a molecular level. In the example of plastic and since it is a nonpolar substance; water will tend form hydrogen bonds with itself. In the example of glass which is polar; water molecules will form hydrogen bonds with the silicon dioxide of the glass. And this is what makes water tend to spread on the glass surface; attraction to glass. Read more about polarity.
Another example explaining surface tension, using a small glass tube called a capillary tube. If we place the end tip of this tube in a glass of water, water will drawn. This is because of the hydrogen bond occurring between the SiO2 of the glass tube and the Oxygen atoms in the water molecules. The definition of hydrophilic which is what describes the glass in this situation means water loving. While definition for Hydrophobic which is plastic in the above example means water hating.
surface tension capillary tube example water glass hydrophilic
A unit of measurement for the surface tension of water is dynes/cm at room temperature. Which is the force you have to overcome in order to break the surface tension of a water droplet (1cm in length). The lower the temperature the less surface tension. Hot water makes a better cleaning solution since it is considered a better wetting agent. This is due to low surface tension of hot water which allows it to better react with detergents which are hydrocarbons with a polar end called a functional group.
- Published in Water Chemistry, Water Treatment
Manganese and Iron in Water
Manganese and Iron in water have secondary MCLs (Maximum Contaminant Level) (SMCLs – Secondary Maximum Contaminant Level) of 0.05 and 0.3 mg/L, respectively. These SMCLs have been considered as safe limits to avoid the staining of plumbing fixtures and laundry, but experience shows that lower levels are desired to avoid difficulties. Targets of < 0.1 mg/L iron and < 0.02 mg/L manganese should be normal water quality goals.
Dissolved Manganese and Iron in water are normally in the reduced state (Fe II and Mn II) and can be removed by oxidizing to Fe III and Mn IV, where they will precipitate as Fe(OH)3 and Mn(OH)2. Precipitates are subsequently removed in sedimentation and/or filtration steps. Several oxidants are available for this process, namely, chlorine, chlorine dioxide, ozone, and potassium permanganate. They are also removed through conventional lime softening treatment. Most oxidants also react with organics in the water, so some testing is normally required to determine the appropriate dose.
Small well treatment water systems with excessive levels of iron and manganese often apply an oxidant, provide a period of detention for the reaction to take place, and then remove the precipitated Manganese and Iron in water with a pressure filter.
Cheapest way to remove iron from well water:
As discussed above, cheapest way to remove iron from well water is to use Chlorine as an effective oxidant for iron removal. Oxidation to Fe(OH)3 proceeds rapidly which is a Ferric Fe3+ Hydroxide (Iron III Hydroxide). Which is a precipitate of Red/Brown color that can be easily removed by a conventional cartridge filter (sedimentation/filtration). It is not as effective for manganese removal, however, at normal pH conditions. Chlorine is also used to maintain MnO2(s) coatings on filter media, which allows for the continuous removal of manganese through the filter bed.
Potassium permanganate, KMnO4, has often been used at treatment plants for oxidation of these two dissolved species. Potassium permanganate is best used at the front of the treatment works to allow contact prior to the introduction of other chemicals. The reactions have been found to be both pH- and temperature-dependent. Permanganate is also used for the regeneration of Manganese Green Sand filter media.
Green Sand Filter iron in water removal
Ozone has been used for Manganese and Iron removal from water because it is effectively used for iron and manganese oxidation. Often in conjunction with protection against tastes and odors. Iron is almost instantaneously oxidized by ozone while manganese is somewhat slower, but even this reaction is normally complete within about 30 seconds.
Oxidation using chlorine dioxide is generally complete within a few seconds. Chlorine dioxide is also normally applied at the head of the treatment process. It has the advantage over chlorine of not forming chlorinated DBPs (Disinfection by-products), but a regulated DBP, chlorite, is one of its by-products. Depending on the concentrations of Manganese and Iron in water, the chlorite formed may limit the use of chlorine dioxide for this application unless the chlorite is subsequently removed by another process.
Lime softening is also an effective way for Manganese and Iron removal. Again, the reduced forms are oxidized to insoluble precipitates, and they are removed along with the lime sludge. The chemical reaction that describes this oxidation process is as follows:
4Fe(HCO3)2 + 4Ca(OH)2 + 2H20 + O2 –> 4Ca(HCO3)2 + 4Fe(OH)3
- Published in Water Treatment
What is Forward Osmosis
Fundamentally, Forward Osmosis exploits a naturally occurring phenomenon (Osmosis). Which is simply the water transport over a semi-permeable membrane from a low concentration to a high concentration. In a perfect world, the semi-permeable membrane permits water to pass through it however rejects all salts and undesirable components. The high salinity solution acts as the draw solution, which has a higher concentration than the feed water. To initiate the passage and attract water to pass through the membrane from the feed side to itself. In this manner, Forward Osmosis requires less energy (applied pressure) to transport a water stream through the membrane. In comparison to the pressure driven membrane procedures, for example, reverse osmosis (RO). However, as opposed to Reverse Osmosis, the result of Forward Osmosis is not fresh water but rather a diluted draw solution. It is a blend of the feed water and draw solution. In this way, a following filtration or separation process must be performed to extract clean water and to recover the draw solution. The draw solution consists of either a single or multiple simple salts or can be a substance specifically tailored for forward osmosis applications.
The following step of filtration might be energy intensive relying upon the draw solution and the recycling procedure. Hence, for potable water production, one must consider the energy utilization of both the Forward Osmosis procedure and the DS recovery. So that we can eventually make a reasonable examination and run comps between Forward Osmosis and other water treatment advancements. Or else, the conclusion could be one-sided and deceiving. In any case, Forward Osmosis might be more practical than pressure driven membrane technologies for water reuse. Only if the recovery of the Draw Solution is not required. Consequently, Researches and developments made on Forward Osmosis will be used to organize those procedures and applications without reusing the Draw Solution.
One of the most significant applications for Forward Osmosis is done by NASA. Six forward osmosis kits will fly aboard space shuttle Atlantis on the STS-135 mission by NASA. Scientists from NASA’s Kennedy Space Center in Florida plan to test a space adapted version of a Forward Osmosis bag. This will happen aboard space shuttle Atlantis during the STS-135 mission summer 2017. The group at Kennedy, led by NASA Project Manager Spencer Woodward, will include in the shuttle’s cargo six forward osmosis bag kits for the astronauts to test. The bags’ manufacturer, made a few adaptations to their commercial product for spaceflight. The idea is to make a fortified drink that provides hydration and nutrients from all sources available aboard a spacecraft, such as wastewater and even urine.
Forward Osmosis bag kit NASA experiment
Up until today, Forward Osmosis still experiences issues as a cost-effective innovation for direct seawater desalination. As a result of its high energy utilization and absence of efficient DS with -close to – negligible reverse flux. Regardless of many innovative advances in DS recently made, challenges still exist to: First, limit the reverse flux of DS. Secondly, mitigate internal concentration polarization (ICP) and Lastly, find better and easier recovery strategies. In conclusion, Forward Osmosis represents a forthcoming technology with a very low impact on the environment.
- Published in Technology, Water Treatment
What is Electrodialysis ED
In electrodialysis (ED)–based desalination systems, the separation of minerals and product water is achieved through the application of direct electric current to the source water. This current drives the mineral ions and other ions with strong electric charge that are contained in the source water through ion-selective membranes to a pair of electrodes
of opposite charges. As ions accumulate on the surface of the electrodes, they cause fouling over time and have to be cleaned frequently in order to maintain a steady-state Electrodialysis ED process. A practical solution to this  challenge is to reverse the polarity of the oppositely charged electrodes periodically (typically two to four times per hour) in order to avoid frequent electrode cleaning.
An Electrodialysis ED process that includes periodic change of the polarity of the system’s electrodes is referred to as an electrodialysis reversal EDR process. At present, practically all commercially available ED systems are of the EDR type. Electrodialysis ED systems consist of a large number (300 to 600 pairs) of cation and anion exchange membranes separated by dilute flow dividers (spacers) to keep them from sticking together and to convey the desalinated flow through and out of the membranes. Each pair of membranes is separated from the adjacent pairs above and below it by concentrate spacers which collect, convey, and evacuate the salt ions retained between the adjacent membranes. The membranes used for ED are different from those applied for Reverse Osmosis RO desalination—they have a porous structure similar to that of microfiltration and ultrafiltration membranes. Reverse Osmosis membranes do not have physical pores. Electrodialysis ED membranes are more resistant to chlorine and fouling and are significantly thicker than RO membranes.
electrodialysis ED membrane desalination technology schematic process system
It is important to note that a single set of EDR stacks can only remove approximately 50 percent of salts. As a result, multiple EDR stacks connected in series are often used to meet more stringent product water Total Dissolved Solids TDS targets. It should be pointed out that compared to brackish water RO membranes, which typically yield only up to 85 to 90 percent recovery, Electrodialysis Reversal EDR systems can reach freshwater recovery of 95 percent or more. The energy needed for ED desalination is proportional to the amount of salt removed from the source water. TDS concentration and source water quality determine to a great extent which of the two membrane separation technologies (RO or ED) is more suitable and cost effective for a given application. Typically, ED membrane separation is found to be cost competitive for source waters with TDS concentrations lower than 3000 mg/L. This applicability threshold, however, is a function of the unit cost of electricity and may vary from project to project.
The TDS removal efficiency of Electrodialysis ED desalination systems is not affected by non-ionized compounds or objects with a weak ion charge (i.e., solids particles, organics, and microorganisms). Therefore, ED membrane desalination processes can treat source waters of higher turbidity and biofouling and scaling potential than can RO systems. However, the TDS removal efficiency of ED systems is typically lower than that of Reverse Osmosis systems (15.0 to 90.0 percent versus 99.0 to 99.8 percent), which is one of the key reasons why they have found practical use mainly for brackish water desalination. In general, Electrodialysis Reversal EDR systems can only effectively remove particles that have a strong electric charge, such as mono- and bivalent salt ions, silica, nitrates, and radium. EDR systems have a very low removal efficiency with regard to low-charged compounds and particles—i.e., organics and pathogens. Table below provides a comparison of the removal efficiencies of distillation, ED, and RO systems for key source water quality compounds.
| Contaminant | Distillation (%) | ED/EDR (%) | RO (%) |
| TDS | >99.9 | 50-90 | 90-99.5 |
| Pesticides, Organics/VOCs | 50-90 | <5 | 5-50 |
| Pathogens | >99 | <5 | >99.99 |
| TOC | >95 | <20 | 95-98 |
| Radiological | >99 | 50-90 | 90-99 |
| Nitrate | >99 | 60-69 | 90-94 |
| Calcium | >99 | 45-50 | 95-97 |
| Magnesium | >99 | 55-62 | 95-97 |
| Bicarbonate | >99 | 45-57 | 95-97 |
| Potassium | >99 | 55-58 | 90-92 |
One important observation from this table is that, as compared to distillation and RO separation, ED desalination only partially removes nutrients from the source water. This fact explains why EDR is often considered more attractive than RO or thermal desalination (which remove practically all minerals from the source water) if the planned use of the desalinated water is for agricultural purposes—i.e., generating fresh or reclaimed water for irrigation of agricultural crops.
Construction and equipment costs for brackish water reverse osmosis (BWRO) and EDR systems of the same freshwater production capacity are usually comparable, or EDR is less costly, depending on the Reverse Osmosis membrane fouling capacity of the source water. However, since the amount of electricity consumed by EDR systems is directly proportional to the source water’s salinity, at salinities of 2000 to 3000 mg/L the energy use of EDR systems usually exceeds that of BWRO or nanofiltration systems for source waters. Therefore, EDR systems are not as commonly used as RO systems for BWRO desalination and are never applied for seawater reverse osmosis (SWRO) desalination.
It should be pointed out, however, that salinity is not the only criterion for evaluating the cost competitiveness of EDR and BWRO systems. Often, other compounds such as silica play a key role in the decision making process. For example, at the largest operational EDR plant worldwide at present—the 200,000 m3/day Barcelona desalination facility in Spain—this technology was preferred to BWRO desalination because the brackish surface water source for this plant—the Llobregat River—contains very high level of silica, which would limit recovery from a BWRO plant to only 65 percent; the EDR system can achieve 90 percent recovery. In addition, the Llobregat River was found to have very high organic content, which was projected to cause heavy fouling and operational constraints on a BWRO plant of similar size.
Reference: “Desalination Engineering” by Nikolay Voutchkov
- Published in Technology, Water Treatment
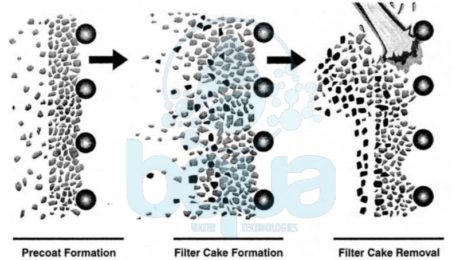
What is Diatomaceous Earth DE Filter
Diatomaceous Earth DE filtration – DE Filter –  has been used effectively for drinking water treatment since 1942. Diatomaceous Earth DE Filter was adopted as a standard method for the U.S. Army. The DE filter was selected because of its portability and effectiveness in removing Entamoeba histolytica cysts (Black and Spaulding, 1944). These cysts are pervasive in some parts of the world and are difficult to control with disinfectants alone. The capability of DE filter to effectively remove particulates applies equally well to the latter concerns of Cryptosporidium and Giardia. Where cyst removals of approximately 6 logs have been achieved (Ongerth and Hutton, 1997). DE filter is commonly called a pre-coat filter because of the pre-coat of filter leaves that initiates every operating cycle.
Although DE filter has DE as the most common pre-coat material used, other pre-coat material such as ground perlite performs as well in many applications. For many years, the type of equipment available limited the use of a Diatomaceous Earth DE filter for municipal drinking water treatment. The use of stainless steel and plastics in the fabrication of equipment has significantly changed the performance capability of the filters by improving their ease of operations and maintenance. DE Filter named upon Diatomceous Earth which is mined from the fossilized remains of microscopic plants called diatoms, deposited in what were the beds of ancient oceans. A powdered medium is manufactured from the diatomite deposits that is almost pure silica. One of the more common diatomite media used for drinking water treatment has a mean particle size of 22.3/xm with 80% of the particles ranging in size from 5 to 64 p~m. This medium, when deposited on the filter septum, has an average pore size of about 7.0/xm.
Diatomaceous Earth Filter Operation
As illustrated below, Diatomaceous Earth DE filter operations occur in three steps:
1. A precoat of about % in. (3 mm) is deposited on the filter.
2. After the precoat has been deposited, filtering begins. And at the same time a small amount of Diatomaceous Eearth material (called body feed) is added to the source water. This is to maintain the porosity of the media.
3. Particulates in the source water are trapped in the pre-coat layer until it reaches maximum head loss. At which time the filter run is terminated and media material is cleaned from the septa.
diatomaceous earth DE filter operation design
Porosity Control of DE Filter
The principal requirement for maintaining effective Diatomaceous Earth DE filter runs is to maintain the porosity of the filter cake. Source water solids generally vary in size and are mixture of relatively inert matter and solids that are predominantly organic. If source water is filtered through the pre-coat alone, the buildup of solids and compression of the accumulated cake quickly reduces DE filter cake porosity. And head loss increases at an exponential rate. This may be avoided by adding body feed to the source water in sufficient amounts to produce a constant flow versus head loss relationship. Although the rate of flow does not affect effluent quality or turbidity breakthrough, the flow rate for pre-coat filters should generally be limited to about 2 gpm/ft 2 (7.8 m/h). The shape of the pre-coat filter head loss curve that reflects both feed and flow conditions is. Therefore, an important feature to control effective filter run performance.
Supplementary Treatment for Diatomaceous Earth DE Filter
Supplementary measures are added to the basic DE filter process to enhance the filtration process and to expand the process to remove some non-particulate constituents. Natural color in source water supplies is caused by either organic or mineral matter. Color results from the decay of plant matter or from the solubilization of iron in the soil. And in many instances the mineral and organic matter are bound together. Therefore color is present either in particulate form or in solution. Particulate color consists mostly of negatively charged colloids, and even though the pre-coat medium has low pore size, charged colloids pass through unless the charge is neutralized.
The use of a strong oxidant such as ozone has been demonstrated to be effective in conditioning color for removal. When color is particulate rather than dissolved, DE filters reduce source water color of about 25 color units (CU) and less to below 5.0 CU. With source color between 25 and 60 CU, filter effluent is generally no higher than 10 CU. Supplemental treatment such as pre-ozonation or alum-coated media may be required. They can improve removal of particulate color and to reduce dissolved color. Dissolved iron precipitates by aeration or by adding a strong oxidant so that the iron is removed as a particulate in DE filtration. The use of magnesite (magnesium oxide) is found to facilitate removal of some forms of iron. Magnesite mixed along with body feed is held for about 10 rain to form negatively charged suspension of magnesium oxide. MgO gradually undergoes hydration and solution.
Manganese may be removed with DE filer with potassium permanganate (KMnO4) added to the body feed. Followed by flow detention, usually from 10 to 20 min. Detention time is important and should be determined in bench and pilot tests. The rate of KMnO4 addition and body feed rate depend on the amount of manganese in the source water. And on also other water quality characteristics. Where iron and manganese are both present in source water, supplementary conditioning must usually be accomplished in separate steps. With iron treatment preceding the KMnO4 addition. When there is a large amount of iron to be removed, it may be necessary to have two filters in series. With iron conditioning preceding the first filter and the addition of KMnO4 and detention between the first and second filters.
Practically the only carryover of solids in DE filter effluents would be very fine Diatomaceous Eearth particles used in pre-coat and body feed. Although the presence of these particles is innocuous to health, effluent water turbidity must meet the established requirements. Use of a finer Diatomaceous Eearth for pre-coat can sometimes achieve lower turbidity levels. A slight reduction in the applied flow rate can also help to improve effluent turbidity. Where only slight additional turbidity reduction is needed. The use of a simple cartridge filter following the DE filter accomplishes the desired DE removal. Use of cartridge filters to polish the effluent will be more effective for the lower-capacity DE installations when solids carryover is minimal. Note that without the addition of separate, additional treatment processes, DE filtration will not reduce the organic content of source water.
You can learn more about the Diatomaceous Earth DE Filter Design
Reference: Water Treatment Plant Design
- Published in Technology, Water Treatment
What is Diatomaceous Earth DE Filter Design
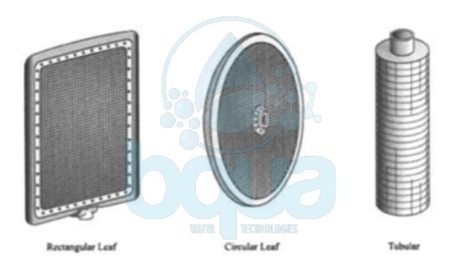
Several options are available in designing each of the flat-leaf DE filter elements, as well as in the integrated assembly DE filter design.
diatomaceous earth DE filter design flat leaf element
Types of DE Filter
Two basic groups of DE filter are available. If source water is to be forced through the filter under pressure, the containment vessel must be closed. DE filter operates under a vacuum, on the other hand, may be open vessels. Although there is theoretically no limitation to what pressure may be applied to a pressure-type filter. Practical considerations of pumping costs have limited head loss to a maximum of 35 psi (241 kPa). Most systems used for drinking water filtration are designed for a maximum head loss of 25 psi (172 kPa).
DE Filter Design Construction
Pressure filters are always constructed as cylindrical pressure vessels mounted either vertically or horizontally. Most units fabricated today are made of stainless steel for the shell and most internal parts. The type of stainless steel used depends on the corrosivity of the water being treated. Vacuum filters are built as rectangular tanks. Because of the low differential heads they are subject to, vacuum filter containments and internal parts, except certain structural supports, are most often fabricated of plastics for their chemical resistance and reduced maintenance. For larger units, the containment vessel may be constructed of concrete.
DE Filter Elements
Inlet water flow is introduced to a DE filter through the containment wall, fitted with an internal baffling device to prevent disturbance of the filter cake. Filter cake may be cleaned from the filter by scraping, vibration, hydraulic bumping (surging), or manually hosing down the septa from the top of an open vessel. Many arrangements of filter elements are available, constructed in both a tubular and flat form, with the flat (or “leaf’) design being by far the most common.
DE Filter elements may be mounted either horizontally or vertically, and they may be either fixed in position or able to rotate. Vertical mounting of the leaves is used almost exclusively for water treatment applications. Most pressure and vacuum filters constructed today have fixed leaves mounted by means of spigot-type “push-on” outlets installed in sockets on a manifold and sealed by O rings or flat gaskets. The outlet manifold is usually located below the leaves to provide them with support while allowing gravity to assist in seating the push-on connections.
Variations for both leaf connections and manifold location are selected depending on operating conditions and requirements for inspection and maintenance. Fixed-leaf pressure filters may also be divided into retracting shell and retracting bundle types for internal access. Both options may be used for any size filter, but the retracting bundle type is is generally preferred for larger units. The retracting shell or tank design has the shell mounted on wheels on rails. An electric motor or hydraulic piston opens and closes the unit.
In the retracting bundle design, the shell head is suspended from an overhead monorail. The bundle, attached by the manifold and frame to the head, is retracted by means of the monorail, which is usually motor-driven. Internal rails attached to the shell support the end of the leaf bundles when the shell is opened. Thorough cleaning of the septa at the end of a filter run is important in maintaining peak efficiency. Most units used for water treatment sluice the cake with water sprays, which creates a slurry that can be easily handled and treated and does not require opening the filter vessel.
Fixed-leaf filters are usually cleaned with high-pressure spray jets mounted on oscillating spray heads, with single or multiple jets directed between the filter leaves. Rotating filter leaves usually have a stationary spray header, and coverage is obtained as the leaves rotate past the sprays. Open filters may be cleaned manually using high pressure sprays and may require covers over the units to contain the spray. Additional devices that may assist in the complete removal of the cake slurry from the filter containment include spray jets in the invert of the vessel or an air scour to suspend the material before the vessel is drained.
diatomaceous earth DE filter design rectangular circular tubular element
Leaf DE Filter Design
The flat DE filter leaf with a broad surface and limited thickness should be designed with the following goals:
• Leaf and outer frame must be stiff enough to resist warping under the force exerted at maximum differential pressure.
• The unit must have a backing screen to prevent the cloth septum from flexing under gradually increasing pressure.
• The path provided for filtrate flow must not restrict flow and must create minimal head loss through the leaf. It is essential that the filter cake remain undisturbed.
An adequate filter leaf design prevents the possibility of cake movement due to warping of the frame or flexing of the septum.
Central Drainage Chamber
There are three basic types of filter leaf drainage chambers. Heavy wire mesh with wire spacing up to 1 in. (2.5 cm); expanded metal sheets that provide a deeper chamber with increased rigidity. And the Trislot, a proprietary design having thin metal bars with welded transverse round or wedge-shaped wires.
DE Filter Backing Screen
The backing screen is an intermediate screen used when the irregular surface of the central drainage chamber may permit flexing of the cloth septa under conditions of varying pressure.
DE Filter Septum
Filter septum materials are cloth weaves made with either stainless steel wires or plastic monofilaments. The principal purpose of the septum is to retain the pre-coat, which must bridge the openings in the weave. Because openings in the weave are larger than the major portion of particulates in pre-coat material, the pre-coat is retained by bridging. The cloth septum must be uniformly woven to produce an even pre-coat that reduces the extent of the recirculation required to deposit the material. The weave must also be designed so that it sluices cleanly, drops the cake freely, and resists plugging and damage. One of the more common wire cloths for water filtration is a standard 24 × 110 Dutch weave. Another type of weave is the multibraid, composed of bundles of wire in both directions. This weave is less vulnerable to the entrapment of particles and blinding than the standard weave. Woven wire cloth may also be “calendered,” which involves passing the cloth through compression rollers to flatten the rounded wire at the surface of the weave. Calendering improves pre-coat retention characteristics and generally strengthens the cloth against rough treatment. Plastic cloth is used predominantly for vacuum-type DE filters and is available in a variety of weaves using either polyester or polypropylene monofilament. Plastic cloth may be supplied as a bag to envelop a filter leaf or as a cloth caulked into a leaf frame.
Binding Frame Closures
The binding frame surrounds the filter leaf to prevent leakage around the septum. The outside frame is also the principal structural element to provide rigidity and prevent warping. Depending on the shape, the outside binder may also collect flow from the central chamber and supplement flow routing to the outlet nozzle.
Vacuum Filter Leaves
Vacuum filter leaves used for drinking water treatment are often made of plastic. The central drainage chamber and outlet spigot are molded in a single piece, usually of high-impact styrene. Ridges or other raised patterns provide the required flow path. The raised pattern is spaced so that intermediate screens are not required. The septum, in the form of an envelope with zipperlike closures, is sealed at the bottom outlet by a gasket that also provides tight closure for the manifold connection.
Outlet Connections
Fixed-leaf filter leaves usually have spigot-type outlet connections made of castings machined to fit into the sockets of the outlet manifold. The central drain hub is used for rotating leaves and is of two-piece construction, clamped to the center of the circular leaf by bolts. The leaf outlet connection must be of sufficient size to allow full flow of the filtrate collected by the leaf at a minimum head loss. As leaf size and loading increase, the distribution of flow within the leaf and the transition to the outlet connection increase in importance.
Reference: Water Treatment Plant Design
- Published in Technology, Water Treatment
What is Osmotic Pressure
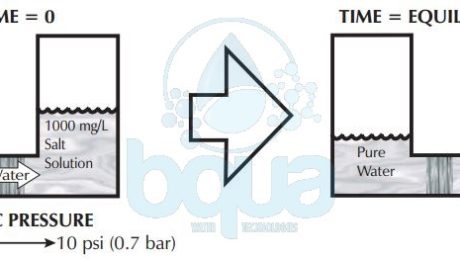
Osmotic pressure is a function of dissolved substances. As in Osmosis, water will pass through a semi-permeable membrane from the lower concentration compartment into the higher concentration compartment. Osmotic pressure is simply what makes osmosis process occurs. The force of this flow is measured by measuring how much pressure must be applied to the higher salt concentration side in order to stop osmosis. This pressure then must be the force of osmosis. This pressure is called osmotic pressure. Osmotic pressure is a function of the number of collisions of water molecules with either side of the membrane. The more dissolved substances, of any kind, there are, the fewer the water collisions.
A very rough rule of thumb is that for every 100 mg/L or ppm of TDS (Total Dissolved Solids) the osmotic pressure is roughly 1 psi (0.07 bar). If pure water is separated from a 1000 mg/L TDS solution by a semi-permeable membrane. It will take around 10 psi of pressure on the 1000 mg/L side in order to stop osmosis. Therefore, the osmotic pressure forcing water from the pure water side into the 1000 mg/L side is 10 psi. While the greater water collisions are from the pure water side of the membrane, the reason for water movement left to right is due to the higher salt concentration on the right hand side.Â
osmotic pressure is what makes osmosis process occurs
Amount of osmotic pressure generated, therefore, is directly proportional to the amount of total dissolved solids (TDS) in the solution. Since every 100 mg/L creates around 1 psi (0.07 bar) of osmotic pressure, we simply have to divide the TDS by 100 (move the decimal to the left two places) in order to calculate the approximate pressure.
Now what happens when we have salt solutions on both sides of a semi-permeable membrane? Net osmotic pressure becomes important. The net pressure is the difference between the pressure of each of the solutions separated by a semi-permeable membrane. If two 1000 mg/L salt solutions separated by semi-permeable membrane, roughly 10 psi of osmotic pressure is being exerted by each solution. In this case the pressures are equal and opposite in direction. Substracting one from the other, the net osmotic pressure is zero. This is when the osmosis process reaches equilibrium.
- Published in Technology, Water Treatment
What is a Cellulose Acetate Membrane
The thin semi-permeable film of the first Reverse Osmosis membranes was developed in the late 1950s at UCLA (University of California Los Angeles). It was made of cellulose acetate (CA) polymer. A Cellulose Acetate membrane have a three-layer structure similar to that of a Polyamide Thin Film Composite TFC membrane. The main structural difference is that the top two layers (the ultrathin film and the microporous polymeric support) are made of different forms of the same Cellulose Actetate polymer.
Cellulose is a polymer that is made up of repeating units (monomers) of C6H10O5. (Note: A monomer is a molecule which comes together with other identical monomers to form a chain of monomers, called a polymer). The number of acetates on the cellulose molecules affects the semi-permeability and other characteristics of the membrane. In general, the following are some of the most important differences between diacetate and triacetate membranes.
cellulose polymer cellulose acetate membrane
In a TFC membrane these two layers are made of completely different polymers. The thin semi-permeable film is polyamide, while the microporous support is polysulfone. Similar to a TFC membrane, a Cellulose Acetate membrane have a film layer that is typically about 0.2 um thick. But the thickness of the entire membrane (about 100 um) is less than that of a TFC membrane (about 160 um). One important benefit of a Cellulose Acetate membrane is that the surface has very little charge and is practically uncharged. As compared to a TFC membrane, which have a negative charge and can be more easily fouled with cationic polymers. If such polymers are used for source water pre-treatment.
cellulose acetate membrane pores under microscope
In addition, a Cellulose Acetate membrane have a smoother surface than a TFC membrane. Which also renders them less susceptible to fouling. Cellulose Acetate membrane have a number of limitations. Including the ability to perform only within a narrow pH range of 4 to 6 and at temperatures below 35 C (95 F). Operation outside of this pH range results in accelerated membrane hydrolysis, while exposure to temperatures above 40 C (104 F) causes membrane compaction and failure. In order to maintain the Reverse Osmosis concentrate pH below 6, the pH of the feed water to the cellulose acetate membrane are reduced to between 5 and 5.5. This results in significant use of acid for normal plant operation and requires Reverse Osmosis permeate adjustment by addition of a base (typically sodium hydroxide) to achieve adequate boron rejection.
Cellulose Acetate membrane experience accelerated deterioration in the presence of microorganisms. Since they’re capable of producing cellulose enzymes and bioassimilating the membrane material. However, they can tolerate exposure to free chlorine concentration of up to 1.0 mg/L. Which helps to decrease the rate of membrane integrity loss due to destruction by microbial activity.
Since Cellulose Acetate membrane have a higher density than a Polyamide TFC membrane. They create a higher headloss when the water flows through the membranes. Therefore a cellulose acetate membrane operates at higher feed pressures, which results in elevated energy expenditures. Despite their disadvantages, cellulose acetate membrane have high tolerance to oxidants (chlorine, peroxide, etc.). As compared to a PA TFC membrane. Cellulose Acetate membrane are used in municipal applications for saline waters with very high fouling potential. Mainly used in the Middle East and Japan in seawater reverse osmosis plants, and for ultrapure water production in pharmaceutical and semiconductor industries.
- Published in Technology, Water Treatment
Chemical Reaction Definition
A Chemical Reaction is the change that occur to a chemical compound or molecule to form another. This usually leads to a change in the arrangement of electrons and breaking of bonds between atoms or molecules. In addition, chemical reaction produces no change to the cores of the atoms involved. New formed substances have different characteristics and properties.
We know how atoms bond together to form molecules, but you may ask why do certain molecules form. Certain elements combine to form compounds or why do certain compounds react with one another to form new compounds.
Chemical Reaction Examples
For example, when we mix calcium carbonate and hydrochloric acid, we get a violent chemical reaction which releases a large amount of gas.
CaCO3 + 2HCl ——> CaCl2 + H2O + CO2
Calcium Carbonate + Hydrochloric Acid -> Calcium Chloride + Water + Carbon Dioxide
In fact, this equation presents a chemical reaction. In this chemical reaction calcium carbonate reacts with hydrochloric acid to form calcium chloride, water and carbon dioxide. Consequently, this along with many other chemical reactions, are considered a spontaneous chemical reaction. Hence, it will begin and continue to completion on its own.
Types of Chemical Reactions
Spontaneous Reactions
2Na + 2H2O ——> 2NaOH + H2
Metallic Sodium + Water -> Sodium Hydroxide + Hydrogen Gas
NaHSO3 + HOCl ——> NaCl + H2SO4
Sodium Bisulfite + Hypochlorous Acid -> Sodium Chloride + Sulfuric Acid
Other chemical reactions are not spontaneous, however, will still continue on its own once started with a type of energy input.
Chemical Reaction Requiring An Initial Energy Input
2H2 + O2 —Energy—>Â 2H2O
Hydrogen Gas + Oxygen Gas -Energy-> Water
CH4Â + 2O2 —Energy—>Â CO2 + 2H2O
Methane + Oxygen -Energy-> Carbon Dioxide + Water
Much as a spontaneous chemical reaction, another type of chemical reaction requiring a continuous energy input.
Chemical Reaction Requiring AÂ Continuous Energy Input
N2 + 3H2 —Cont. Energy—>Â 2NH3
Nitrogen Gas + Hydrogen Gas -Cont. Energy-> Ammonia
CaCO3 —Cont. Energy—>Â CaO + CO2
Calcium Carbonate -Cont. Energy-> Calcium Oxide + Carbon Dioxide
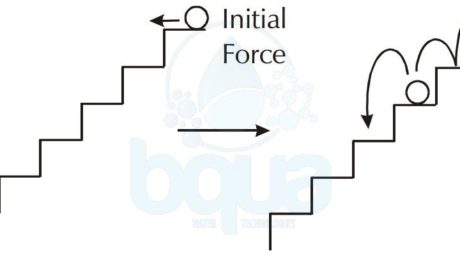
In addition, can actually explain the chemical reaction types using the example of a ball rolling down stairs. In the case of the spontaneous chemical reaction, we can see the ball rolling down a set of stairs where each step is slightly sloped downhill. What happens is that the ball continues to roll down from step to step with no assistance.
spontaneous chemical reaction example ball rolling down set of inclined stairs
As in the case of a reaction requiring an initial energy input, we can use a ball rolling down a set of stairs where each step is a level. The ball continues to roll from step to step by an initial input trigger which is a push.
chemical reaction initial energy input example ball rolling down stairs
In the last example, a chemical reaction requiring continuous energy input, we can imagine a ball starting from the bottom of the stairs. In order to get the ball to the top, we have to continually add energy to the reaction.
chemical reaction continuous energy input example ball from bottom to top of stairs
As in the case with the ball and stairs, a spontaneous chemical reaction will proceed in the direction
which produces products having the lowest potential energy level. Furthermore, the chemical reactions which require only an initial energy boost have an energy “hump†which must be overcome before the reaction will proceed on its own.
Finally, the non-spontaneous chemical reaction produces products having a higher potential energy level. Therefore, these reactions require a continuous energy input (rolling the ball uphill). The change in energy between the reactants and products of a reaction is referred to as the change in enthalpy.
- Published in Water Chemistry, Water Treatment
What is a Hydrogen Bond
A water molecule will form a bond called a hydrogen bond. A hydrogen bond is formed because of the polarity of the molecule and the fact that hydrogen atoms are present in the molecule. water molecules will form hydrogen bond with another molecule(s) of water. These bonds are not nearly as strong as the covalent bond between the hydrogen atoms and the oxygen atom within one H2O molecule.
We can simply say that a Hydrogen Bond is a weak bond that occurs between a proton of a molecule and an electronegative atom of another molecule as a result of an electrostatic attraction between the two molecules. The Hydrogen bond can either happen between two different molecules or within the same molecule.
Hydrogen bond gives water many of its amazing properties, including:
– High Boiling Point
–Â Surface Tension
– Capillary Action
– Solvent Ability
The molecular weight of water is 18; which is the sum of the atomic weights of the atoms in one molecule. Most compounds of such a small molecular weight (MW) are gases at room temperature and pressure.
Considering the example of nitrogen (N2) as compared to water. Nitrogen has a MW of 28 and a boiling point of 321°F (-196°C) below zero. Water, on the other hand, has a MW of 18 and a boiling point of 212°F (100°C), a difference of 533°F (296°C).
hydrogen bond water and nitrogen difference properties
The difference between nitrogen and water is that the nitrogen molecule has no force of attraction between molecules. Water, of course, has the hydrogen bond between molecules. Although the hydrogen bonds are weak compared to other bonds, they are significant enough to create the tremendous difference in boiling points between the two compounds. The picture below shows few molecules of water and molecules of nitrogen within a pure liquid state.
hydrogen bonding water nitrogen difference in properties
- Published in Water Chemistry, Water Treatment
What is Polarity Definition
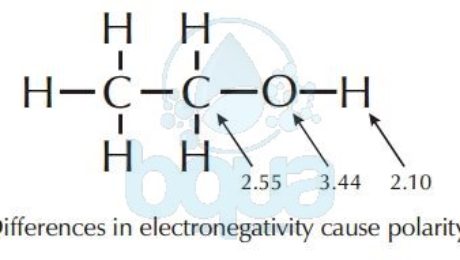
Polarity is basically the difference in electronegativity between two molecules; which is simply the affinity of an atom to electrons. Atoms of the same molecule have influence on each other in many ways. One of the reasons is electronegativity. An example is a water molecule, because of the electronegativity difference between the Oxygen atom (3.4) and Hydrogen atom (2.2) it has areas of partial negative and positive charges. Meaning that one side of the bond possesses a partial negative charge, while the other side has a partial positive charge. This is referred to as polarity, and the water molecule is said to be polar. This polarity is due to the electronegativity of the atom-of-oxygen.
The example of the Oxygen – atom in the water – molecule explains polarity. Oxygen atoms attracts electrons more than the Hydrogen atoms. This results that the pair of electrons shared spend more time around the oxygen nucleus than around the hydrogen nucleus. When a shared electrons’ pair is orbiting the oxygen nucleus, the proton of the hydrogen nucleus is exposed and the oxygen/atom has more electrons than protons. This creates the partial -ve and partial +ve charges making the molecule of water acquire polarity characteristics.
Electronegativity of oxygen cause polarity in water-molecule
The relative electronegativities of the atoms involved is what determines the polarity of bonds determined. These electronegativity values are also determined from a variation of the periodic table of the elements called the periodic table of elements.
Most hydrocarbons (molecules made up of both Carbon and Hydrogen atoms) are extremely nonpolar because of the similarity in the electronegativity of Carbon – Hydrogen. In other words, the hydrogen and carbon atoms share electrons fairly equally.
Polarity caused by electronegativity difference between two atoms in moleculeÂ
Polarity and properties of molecules
Nonpolar molecules do not have significant partial charges, therefore they will not attract polar molecules. Opposite charges attract, similar charges repel one another, but there is no attraction or repulsion between nonpolar molecules. For example, we know that gasoline (a hydrocarbon) will not mix with water. However, if we add oxygen to the hydrocarbon molecules we see that the molecules become polar. Ethanol (drinking alcohol), for instance, completely mixes with water.
Soaps and detergents are unique molecular combinations of polar and nonpolar compounds. Nonpolar part of molecule will react with oils found on soiled hands or clothing while polar part reacts with water. The result is oils removal (nonpolar) from water (high polarity). The polar end of soap is a functional group.
soap made of hydrocarbons and polar end functional group
The incompatibility between nonpolar compounds and water is due to the attraction that water molecules exhibit for one another. Positive charge on the hydrogen of a water-molecule will attract negative charge of atom of oxygen in adjacent water molecule. This attraction, called a hydrogen bond, will cause a type of bond between the molecules themselves.
Because of lack of charges, nonpolar compound won’t have the strength to break the hydrogen bonds between the water molecules. Therefore the nonpolar compound won’t combine with water.
- Published in Water Chemistry, Water Treatment
Water Treatment Abbreviations and Acronyms – Glossary
This page includes a list of water treatment abbreviations and acronyms. It is considered as a small water glossary. Please feel free to contact us if you want to add a missing abbreviation.
| A | Water permeability coefficient |
| Ã… | Angstrom |
| ac | Acre |
| AOC | Assimilable organic carbon |
| AC | Alternating current |
| AC | Acticated Carbon |
| ADI | Acceptable daily intake |
| AO | Assimilable Organic |
| AWA | Australian Water Association |
| AWWA | American Water Works Association |
| B | Beta (concentration polarization factor) |
| B | Boron |
| Ba | Barium |
| BF | Bioflocculation |
| BF | Bag Filter |
| BOD | Biological oxygen demand |
| BOOT | Build-own-operate-transfer |
| Br | Bromide |
| BWRO | Brackish water reverse osmosis |
| °C | Degree Celsius (unit of temperature) |
| ΔC | Concentration gradient |
| Cf | Contingency factor |
| Ca | Calcium |
| CA | Cellulose acetate |
| CAPEX | Capital Expenditure investment |
| CaCO3 | Calcium carbonate |
| CCPP | Calcium carbonate precipitation potential |
| CCTV | Closed Circuit Television |
| CEDI | Continuous Electrodeionization |
| CF | Concentration factor |
| CF | Cartridge Filter |
| CFD | Computational fluid dynamics |
| cfu | Colony Forming Unit |
| CIP | Clean-in-place |
| cm | Centimeter |
| CMF | Conventional media filtration |
| CO2 | Carbon dioxide |
| COD | Chemical Oxygen Demand |
| CWA | Clean Water Act |
| DAF | Dissolved air flotation |
| DBB | Design-bid-build |
| DBO | Design-build-operate |
| DBP | Disinfection byproduct |
| DC | Direct current |
| DO | Dissolved oxygen |
| DOC | Dissolved organic carbon |
| DW | Dry weight |
| EC | Electrical conductivity |
| ED | Electrodialysis |
| EDR | Electrodialysis reversal |
| EPA | Environmental Protection Agency |
| EPC | Engineering, procurement, and construction |
| ERD | Energy Recovery Device |
| °F | Degree Fahrenheit (unit of temperature) |
| Fp | Feed pressure |
| FAO | Food and Agriculture Organization (United Nations) |
| FRP | Fiberglass-reinforced plastic |
| ft | Foot |
| ft2 | Square foot |
| ft3 | Cubic foot |
| FO | Forward Osmosis |
| Gpm | Gallons per minute |
| gal/min | Gallons per minute |
| gfd | Gallons per square foot per day |
| GAC | Granular Activated Carbon |
| GOR | Gained output ratio |
| GRP | Glass-reinforced plastic |
| GWA | Government of Western Australia |
| GWI | Global Water Intelligence |
| Hr | Hour |
| ha | Hectare |
| HACCP | Hazard Analysis and Critical Control Point |
| HBMP | Hydrobiological monitoring program |
| HC | Hydrocarbon (H-C bond) |
| HDPE | High-density polyethylene |
| HOCl | Hypochlorous Acid |
| Hp | Horsepower (unit of power) |
| H2S | Hydrogen sulfide |
| IDA | International Desalination Association |
| IPP | Industrial pretreatment program |
| IWA | International Water Association |
| IWW | Industrial Wastewater |
| IX | Ion exchange |
| J | Membrane permeate flux |
| JTU | Jackson Turbidity Unit |
| Kg | Kilogram |
| Km | Kilometer |
| kW | Kilowatt (unit of power) |
| kWh | Kilowatt-hour (unit of energy) |
| kWh/m3 | Kilowatt-hours per cubic meter (unit of energy) |
| L | Liter |
| LDPE | Low Density Polyethylene |
| LPM | Liter per Minute |
| lb | Pound |
| lb/in2 | Pounds per square inch (unit of pressure) |
| Lmh | Liters per square meter per hour |
| LSI | Langelier Saturation Index |
| LT2ESWTR | Long Term 2 Enhanced Surface Water Treatment Rule |
| M | Meter |
| m2 | Square meter |
| M3 | Cubic meter |
| M3/day | Cubic meters per day |
| MBF | Mixed-media Bed Filter |
| MBR | Membrane Bioreactor |
| MED | Multi-effect distillation |
| MED-TC | Multi-effect distillation with thermal compression |
| MENA | Middle East and North Africa |
| Meq | Milliequivalent |
| MF | Microfiltration |
| Mg | Magnesium |
| mg/l | Milligrams per liter |
| MGD | Million gallons per day |
| Mi | Mile |
| Min | Minute |
| ml | Milliliter |
| mm | Millimeter |
| Mn | Manganese |
| MSD | Multistage flash distillation |
| MTBE | Methyl tertiary butyl ether (gasoline additive) |
| mV | Millivolt |
| MVC | Mechanical vacuum compression |
| µg/L | Micrograms per liter |
| µm | Micrometer |
| µS/cm | Microsiemens per centimeter (unit of specific conductance) |
| Na | Sodium |
| NaOCl | Sodium Hypochlorite |
| NDMA | N-nitrosodimethylamine |
| NDP | Net driving pressure |
| NF | Nanofiltration |
| NOM | Natural organic matter |
| NP | Nanoparticle |
| NPDES | National Pollutant Discharge Elimination System |
| NTU | Nephelometric turbidity unit |
| O&M | Operations and maintenance |
| Opfc | Osmotic pressure on the concentrate side of the membrane |
| OCSD | Orange County Sanitation District |
| ORP | Oxidation-reduction potential |
| P | Phosphorus |
| Pr | Permeate recovery rate |
| PA | Polyamide |
| PDFB | Percent difference from balance |
| pH | Indication of the acidity or basicity of solution |
| PP | Polypropylene |
| ppb | Parts per billion (1 ppb = 1 µg/L) |
| ppm | Parts per million (1 ppm = 1 mg/L) |
| Ppt | Parts per trillion |
| PSI | Pound per square inch (unit of pressure) |
| PREN | Pitting resistance equivalent number |
| psu | Practical salinity unit (1 psu = 1000 mg/L) |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| Qf | Fresh water flow |
| Rp | Permeate recovery rate |
| RBF | Rapid Bioflocculation Filter |
| RCRA | Resource Conservation and Recovery Act |
| RIB | Rapid infiltration basin |
| RO | Reverse osmosis |
| ROWPU | Reverse Osmosis Water Purification Unit |
| RSF | Rapid Sand Filtration |
| RWQCB | Regional water quality control board |
| s | Second |
| S | Membrane area of an element |
| Sp | Salt passage |
| Sr | Salt rejection |
| S. AZ | Southern Arizona |
| S. CA | Southern California |
| SAR | Sodium adsorption ratio |
| SARI | Santa Ana River Interceptor |
| SBR | Sequencing Batch Reactor (wastewater) |
| SCADA | Supervisory Control and Data Acquisition |
| SDI | Silt Density Index |
| SDWA | Safe Drinking Water Act |
| SDSI | Stiff–Davis Saturation Index |
| SER | Standard evaporation rate |
| SF | Sand Filter |
| SI | International System of Units |
| SRB | Sulfide Reducing Bacteria |
| SMBS | Sodium Metabisulfite |
| STE | Salinity tolerance evaluation |
| SUVA | Specific UV absorbance |
| SWRO | Seawater reverse osmosis |
| TCLP | Toxicity characteristic leaching procedure |
| TDS | Total dissolved solids |
| TFN | Thin-film nanocomposite |
| THC | Total hydrocarbons |
| TMP | Transmembrane pressure |
| TN | Total nitrogen |
| TOC | Total organic carbon |
| TP | Total phosphorus |
| TSS | Total suspended solids |
| u | Atomic mass unit |
| UF | Ultrafiltration |
| UIC | Underground Injection Control |
| US | United States (of America) |
| USBR | U.S. Bureau of Reclamation |
| USDW | Underground source of drinking water |
| UV | Ultraviolet radiation |
| VC | Vapor Compression |
| W. TX | Western Texas |
| WEF | Water Environment Federation |
| WET | Whole effluent toxicity |
| WHO | World Health Organization |
| WPA | Water purchase agreement |
| WQA | Water Quality Association |
| WTP | Water treatment plant |
| WWTP | Wastewater treatment plant |
| Y | Plant recovery |
| ZID | Zone of initial dilution |
| ZLD | Zero liquid discharge |
- Published in Water Treatment
What is Multi Stage Flash Distillation MSF
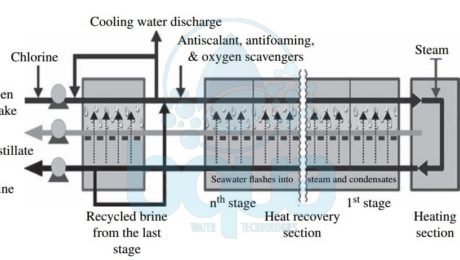
The Multi Stage Flash distillation MSF evaporator vessels is also referred to as flash stages or effects. What happens is that the high-salinity source water is heated to a temperature of 90 to 115°C (194 to 239°F) in a vessel (the heating section in the figure below) to create water vapor. The pressure in the first stage is maintained slightly below the saturation vapor pressure of the water. So when the high-pressure vapor created in the heating section enters into the first stage, its pressure is reduced to a level at which the vapor “flashes†into steam. Steam (waste heat) for the heating section is provided by the power plant co-located with the desalination plant. Each flash stage (effect) has a condenser to turn the steam into distillate. The condensers are equipped with heat exchange tubes, which are cooled by the source water that is fed to the condensers.
schematic multi stage flash distillation process
Entrainment separators (mist eliminators or demister pads) remove the high-salinity mist from the low-salinity rising steam. This steam condenses into pure water (distillate) on the heat exchange tubes and is collected in distillate trays. This is from where it is conveyed to a product water tank. Distillate flows from stage to stage and is collected at the last stage. The concentrate (brine) is generated in each stage and after collection at the last stage some of it typically is recycled to the source water stream in order to reduce the total volume of source water that must be collected by the intake for desalination. The recirculated brine flowing through the interior of the condenser tubes also removes the latent heat of condensation.
As a result, the recirculated brine is also preheated close to maximum operating temperature. Thereby recovering the energy of the condensing vapor and reducing the overall heating needs of the source water. This “brine recycle†feature has been adopted in practically all of the most recent MSF facility designs and allows significant improvement of the overall cost competitiveness of MSF installations. Each flash stage typically produces approximately 1 percent of the total volume of the desalination plant’s condensate. Since a typical MSF unit has 19 to 28 effects, the total MSF plant recovery (i.e., the volume of distillate expressed as a percentage of the total volume of processed source water) is typically 19 to 28 percent. For comparison, Reverse Osmosis seawater desalination SWRO plants have a recovery of 40% to 45%. The latest Multi Stage Flash Distillation technology has 45-stage units—i.e., can operate at 45% recovery. This feature allows it to compete with Reverse Osmosis systems in terms of recovery.
Historically, MSF was the first commercially available thermal desalination technology. It was applied to produce potable water on a large-scale, which explains its popularity. MSF plants represent over 80% of thermally desalinated water today. The gained output ratio GOR for MSF systems is typically between 2 and 8; the latest MSF technology has a GOR of 7 to 9. The pumping power required for the operation of the MSF systems is 2.0 to 3.5 kWh/m3 (7.6 to 13.3 kWh/1000 gal) of product water.
Reference: “Desalination Engineering” by Nikolay Voutchkov
- Published in Technology, Water Treatment
What is Multi Effect Distillation MED
In Multi Effect Distillation MED systems, saline source water is typically not heated; cold source water is sprayed via nozzles or perforated plates over bundles of heat exchange tubes. This feed water sprayed on the tube bundles boils, and the generated vapor passes through mist eliminators. Which collect brine droplets from the vapor. The feed water that turned into vapor in the first stage (effect) is introduced into the heat exchange tubes of the next effect. Because the next effect is maintained at slightly lower pressure, although the vapor is slightly cooler, it still condenses into freshwater at this lower temperature.
This process of reducing the ambient pressure in each successive stage allows the feed water to undergo multiple successive boilings without the introduction of new heat. Steam flowing through the exchange tubes is condensed into pure water and collected from each effect. Heating steam (or vapor) introduced in the heat exchange tubes of the first effect is provided from an outside source by a steam ejector. The Multi Effect Distillation MED system shown in the figure below is also equipped with a brine recycle system. It allows the introduction of warmer-than-ambient water in the first effects of the system. Thereby reducing both the volume of feed water that must be collected by the plant intake system and the overall energy needs of the system.
The main difference between the Multi Effect Distillation MED and Multi Stage Flash Distillation MSF processes is that while vapor is created in an MSF system through flashing, evaporation of feed water in MED is achieved through heat transfer from the steam in the condenser tubes into the source water sprayed onto these tubes.
schematic multiple effect distillation process system
This heat transfer at the same time results in condensation of the vapor to freshwater. Multi Effect Distillation MED desalination systems typically operate at lower temperatures than MSF plants. Maximum brine concentrate temperature of 62 to 75°C versus 115°C) and yield higher GORs.
The newest Multi Effect Distillation MED technologies include vertically positioned effects (vertical tube evaporators). This may yield a GOR of up to 24 kg of potable water per kilogram of steam. Pumping power required for operation of Multi Effect Distillation MED systems is lower than that typically needed for MSF plants. It is equal to 0.8 to 1.4 kWh/m3/3.0 to 5.3 kWh/1000 gallons of product water. Therefore, Multi Effect Distillation MED is now increasingly gaining ground over MSF desalination. Especially in the Middle East, where thermal desalination is still the predominant method for producing potable water from seawater.
Reference: “Desalination Engineering” by Nikolay Voutchkov
- Published in Technology, Water Treatment
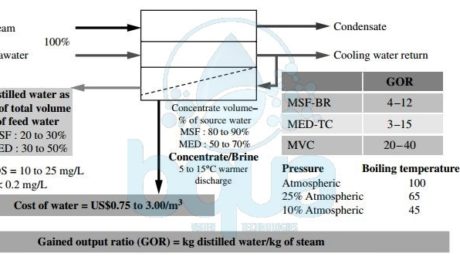
What is Thermal Desalination Technology
All thermal desalination technologies apply distillation. This means heating the source water to produce water vapor, then condense into a low-salinity water. Since the energy for water evaporation is practically not dependent on the salinity concentration of the feed water source, thermal evaporation is very suitable for desalination of high salinity waters and brine. This is one of the reasons that thermal desalination has been widely adopted by Middle Eastern countries. Such countries are Saudi Arabia, Oman, Qatar, the United Arab Emirates, Bahrain and Kuwait. These countries use some of the most saline water bodies on the planet for water supply: Red Sea, Persian Gulf, Gulf of Oman, and Indian Ocean. At present, approximately 75% of the world’s thermal desalination plants are located in the Arabian Peninsula—half of those are in Saudi Arabia.
All thermal desalination plants have five key streams:
1- Source water (seawater, brackish water, or brine) used for desalination.
2- Steam needed for evaporation of the source water.
3- Cooling water to condense the freshwater vapor generated from the source water’s evaporation.
4- Low salinity distilled water (distillate).
5- And concentrate (brine), which contains the salts and other impurities separated from the source water.
The three most commonly used types of thermal desalination technologies are multi stage flash distillation (MSF), multi effect distillation (MED), and vapor compression (VC). Each of these classes of technology has evolved over the past 40-60 years toward improvements in efficiency and productivity. For example, MSF-BR is the abbreviation for a multi stage flash distillation process with brine recycle. MSF-BR reduces the source water volume and the steam needed for evaporation. Similarly, MED-TC stands for multi effect distillation with thermal compression. It is the state-of-the art MED technology. MVC is an acronym for mechanical vapor compression, a VC technology that can run without the need for an outside source of steam.
thermal desalination and evaporation distillation technologies
The three types of thermal technologies mainly differ by temperature and pressure at which source water is boiled to generate freshwater vapor. The oldest thermal evaporation process—MSF—boils water at near atmospheric pressure and a temperature close to 100°C (212°F). This type of process requires a large quantity of high-temperature steam. Multi Effect Distillation MED and Vapor Compression VC are newer thermal desalination technologies. They improved efficiency stems from the fact that water can be boiled at a lower temperature if the boiling process occurs at a pressure lower than the atmospheric pressure. Boiling water at a lower temperature allows the use of less and lower-quality steam for the production of the same volume of water.
In Multi Effect Distillation MED vessels, the boiling process typically occurs at lower temperatures and pressures than in MSF systems. Vapor Compression VC thermal desalination systems operate at lower pressures than either Multi Stage Flash Distillation MSF or MED. which allows these systems to evaporate water at even lower temperatures and to generate their own steam rather than depend on outside steam sources. The ratio of the mass of low-salinity water (distillate) produced to the mass of heating steam used to produce this water is commonly referred to as the gained output ratio (GOR) or performance ratio.
Depending on the thermal desalination technology used, the site-specific conditions, and the source water quality, GOR typically varies between 4 and 40. Thermal desalination technologies produce 4 to 40 kg of freshwater using 1 kg of steam. The higher the GOR, the more efficient the technology, because it produces more freshwater from the same amount of steam. As seen in the figure, all thermal desalination technologies generate very low-salinity water (TDS in a range of 5 to 25 mg/L). This freshwater also has a very low content of pathogens and other contaminants of concern. Such as boron, bromides, and organics. Thermal desalination is most popular in the Middle East, where seawater desalination is typically combined with power generation. Therefore provides low-cost steam for the distillation process. Thermal desalination requires large quantities of steam. Most power plants outside the Middle East are not designed to yield significant amounts of waste steam as a side product of power generation. This is one of the key reasons why thermal desalination has not found wider application outside of the region.
Today, Seawater Reverse Osmosis SWRO dominates the global desalination market in term of capacities. They exceed both traditional thermal technologies (MSF and MED) which are common in the Middle East and North Africa regions. Seawater Reverse Osmosis SWRO is dominating worldwide over thermal technologies in terms of annual installed capacities. Yet the evolution of seawater reverse osmosis into the Gulf and MENA region, particularly in the Gulf of Arabia, has been slow. The reasons are higher feed water salinity affected by hydrocarbons and Red Tide events. Red tide is a phenomenon caused by algal blooms during which algae become so numerous that they discolor coastal waters. The algal bloom may also deplete oxygen in the waters and/or release toxins that may cause illness in humans and other animals.
Reference: “Desalination Engineering” by Nikolay Voutchkov
- Published in Technology, Water Treatment
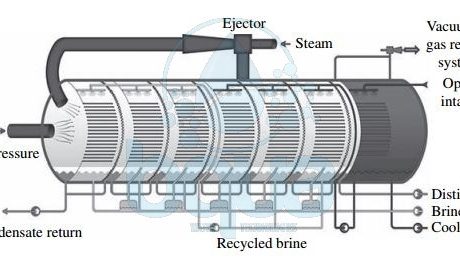
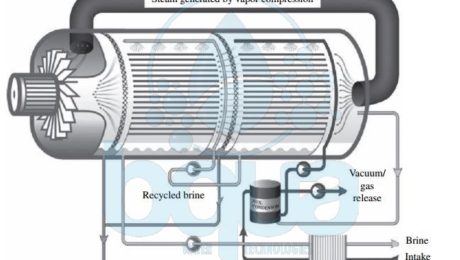
What is Vapor Compression VC
Vapor Compression VC is one type of thermal desalination technologies which also include Muti Stage Flash Distillation MSF and Multi Effect Distillation MED. In Vapor Compression VC, the heat source for vapor compression (VC) systems is compressed vapor produced by a mechanical compressor or a steam jet ejector rather than a direct exchange of heat from steam . In Vapor Compression VC systems the source water is evaporated and the vapor is conveyed to a compressor. The vapor is then compressed to increase its temperature to a point adequate to evaporate the source water sprayed over tube bundles through which the vapor is conveyed. As the compressed vapor exchanges its heat with the new source water being sprayed on the evaporation tubes, it is condensed into pure water. A feed water preheater (plate-type heat exchange) is used to start the process and reach evaporation temperature. Vapor Compression VC and Multi Effect Distillation MED work based on similar principles.
vapor compression tehnology thermal desalination process system
However, in Multi Effect Distillation MED steam produced by source water evaporation is introduced and condensed in a separate condenser. Condenser is located in the downstream effect. In Vapor Compression VC, the steam generated from evaporation of new source water sprayed on the outside surface of the heat exchange tubes is recirculated by the vapor compressor. It is then introduced into the inner side of the of the same heat exchange tubes in which it condenses to form distillate. Vapor Compression VC desalination has found applications mostly in small municipal and resort water supply systems. As well as industrial applications. The total amount of power required for the operation of mechanical Vapor Compression VC systems is typically 8 to 12 kWh/m3 (30 to 45 kWh/1000 gal) of product water.
Reference: “Desalination Engineering” by Nikolay Voutchkov
- Published in Technology, Water Treatment
What is an Atom Definition
An atom is the most discrete indivisible particle that forms an element. Atoms of the same element are identical; different elements have non-similar atoms. The most simple of all elements is Hydrogen.Â
Example: Hydrogen atoms are similar while Oxygen atoms are different than Hydrogen atoms. Two hydrogen atoms and one Oxygen combined form the water molecule H2O.
The atoms are extremely small. Therefore, even the most powerful microscopes cannot see them. Actually, 6.022X10^23 hydrogen atoms (Avogadro Number) weigh only one gram. There are 454 grams in one pound.
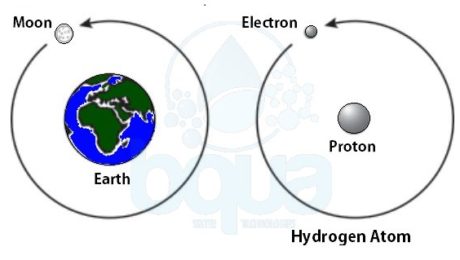
In order to understand the nature of the atom, we should identify the forces that are holding it together. Much as our plant, gravity is the force holding the moon in its orbit around the earth. Gravity is the force of attraction between two large masses.
In the case of the atom, the small size of the particles and the speed at which the electron travels would prohibit a force such as gravity from being able to hold the atom together. Scientists have learned that electrostatic force is the force that maintains the atom.
Atoms Composition
While atoms are composed of small subatomic particles; these particles are the electron, the proton and the neutron. The proton and neutron are found in the nucleus which is the center of the atom. The electron is negatively charged and orbits around the nucleus.
atom composed of subatomic particles electron proton neutron
Atoms from different elements are constituted of the same type of subatomic particles. However, the proportions of the subatomic particles are different for each element. Hydrogen for example has two subatomic particles; an electron and a proton. In the case of hydrogen, a single electron orbits a single proton. ÙAn example is the moon which orbits the earth. Same as in the case of earth, the proton of the hydrogen atom is much larger than the electron.
an electron orbits proton in nucleus in an atom like moon orbits earth
Moreover, if we add a subatomic particle to a Hydrogen atom, we create another atom of another element. Note: The number of subatomic particles is what characterizes atoms of different elements. In addition, if we want to create a Helium atom, we should only add an additional electron, an additional proton and two neutrons to the Hydrogen atom. As a result, we’ll have two electrons orbiting four other particles, two protons and two neutrons in the nucleus.
- Published in Water Chemistry, Water Treatment