What is a Functional Group
Molecular compounds owe their individual properties to functional group. These are combinations of atoms which may appear in various parts of the molecule. A list of major functional group listed below.
| Illustrative Example | Name of Functional Class | Functional Group |
| CH3CH2CH3 | Alkanes | C-C |
| CH3CH=CH2 | Alkenes | C=C |
| CH3CH/CH | Alkynes | C/C |
| CH3CH2CH2OH | Alcohols | C–OH |
| Aldehydes | ||
| Ketones | ||
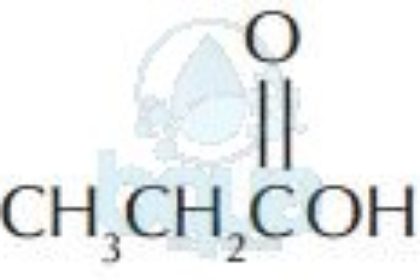
| Acids | ||
| CH3CH2CH2NH2 | Amines | C-NH2 |
| CH3OCH2CH3 | Ethers | -C-O-C- |
| CH3CH2CH2Br | Halides | C-Br |
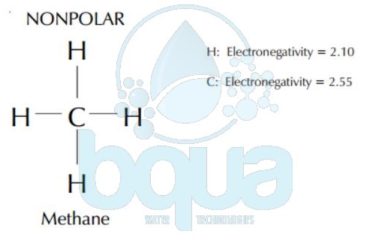
The difference in electronegativity of two bonding atoms determines if the bond will be polar or nonpolar (read about polarity). The bond between two identical atoms is always nonpolar, (for example: C-C, C=C, C=C; H-H, O=O, etc…). Bonds between atoms with electronegativities which are nearly the same are nonpolar (for example C-H). Bonds between atoms with electronegativities which are significantly different (commonly 0.9) are polar (for example, C-O, H-O, C=O, and N-H).
One molecule, especially a large complex molecule, may have more than one type of functional group. One specific functional group may be repeated several times.
A functional group may alter the properties of a compound such as melting point, boiling point, solid structure, and solubility. A functional group on an organic molecule also contribute to its hydrophobic and hydrophilic properties. Surfaces composed of nonpolar molecules will be hydrophobic. Surfaces composed of compounds containing polar bonds will be hydrophilic. You can also read about surface tension.