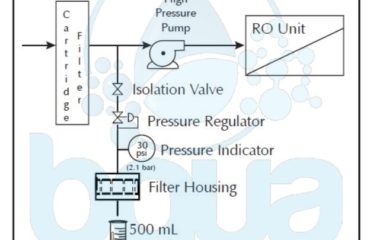
Concentration Polarization
If there are fouling feed water colloidal silica particles, these sub-micron particles will not be trapped in feed water spacer. They may be deposited evenly on the surface of the membrane, causing a problem in all stages. Usually the colloidal particles create more of a last stage problem. Colloidal fouling occurring in the last stage is likely due to agglomeration of sub-micron particles into larger fouling particles due to surface charge neutralization of the particles by double- and triple-charged cations concentrated at the surface of the Reverse Osmosis RO membrane. Due to concentration polarization, colloidal fouling is more likely at the surface of the membrane and in the rear end of a Reverse Osmosis System.
concentration polarization boundary layer on RO membrane surface
Whenever water flows past a solid, there is some attraction and adhesion between the water and the solid. Adhesion between the water and the solid surface causes the water molecules closest to the surface to be stationary relative to it. The farther from the surface water molecules are, the less attraction and adhesion, and therefore the more velocity relative to the surface. Right at the membrane surface, there is no tangential flow. This is the boundary layer When tiny particles deposit on the membrane surface, there is no tangential flow to sweep them off.
concentration polarization on reverse osmosis membrane
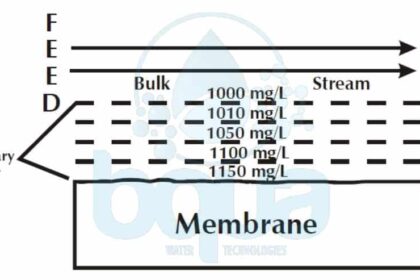
Additionally, the flow of water through the RO membrane, when the high pressure pump is on, will tend to hold them there Not only are feed water particles deposited on the membrane in this way. They may also be grown there. This is likely caused by concentration polarization. Concentration polarization is a function of the boundary layer. It refers to an increased salt concentration at the RO membrane surface. A portion of the feed water passes through the boundary layer and through the membrane.
The water passes through the membrane. The dissolved components do not readily pass through the membrane. The dissolved components, therefore, are left behind at the membrane surface. This causes the salt concentration (TDS, Total Dissolved Solids concentration) to be higher at the membrane surface If the boundary layer had tangential flow and was turbulent, the incoming feed water would mix up the boundary layer and the boundary layer would have the same concentration as the bulk stream.
concentration polarization and total dissolved salts
The boundary layer, however, has no tangential flow. The salts concentrate at the surface. Fortunately, the dissolved substances can diffuse away from the membrane. Recall that diffusion is the movement of dissolved substances from higher concentration to lower concentration. So if we could look into the boundary layer, we would see that, due to diffusion, the salt concentration diminishes from the surface of the membrane to the top of the boundary layer.
At the top of the boundary layer, the concentration is the same as the bulk stream The higher the water flux through the membrane, the more salts collect at the membrane surface, and therefore the higher the salt concentration at the membrane surface. Typically the TDS at the membrane surface is 10% – 15% higher than that measured in the bulk stream. If water flux is increased, a higher TDS will occur at the surface. As we will see below, concentration polarization not only impacts dissolved solids. It may also affect suspended solids.