What is Electronegativity – Electronegativity Definition
The electronegativity determines whether a bond between two atoms is polar or nonpolar which is defined by Polarity. The electronegativity is a measurement of how attractive an atom is to electrons. The more electronegative an element is, the more the shared electrons will spend around that atom. When one atom in a bond gets to keep the electrons more than the other, it assumes a partial negative charge. The other bonded atom has the electrons less often, so assumes a slight positive charge. Like the negative and positive poles of a magnet. One end of the bond is positive and one end is negative, so the bond is said to be polar. Carbon and hydrogen bonds, as in methane, are nonpolar because the electronegativity of both are very close. Carbon and oxygen bonds are polar because the electronegativity of them are substantially different.
Electronegativity example in Methane molecule
Polar substances dissolve in polar substances because the partial positive & negative charges of one molecule are attracted to the partial +ve and -ve charges of other molecules. Nonpolar substances will dissolve in nonpolar substances. This is because of other forces of attraction, such as Van der Waals’ forces, can come into play. Nonpolar compounds generally do not dissolve in polar compounds, and vice versa, because there is no attraction between things that are charged and things that aren’t. Since water is polar, compounds which are polar will generally dissolve well.
Therefore, if we have an organic which is polar, such as methyl alcohol, it will dissolve well in water. In general, the more polar an organic is, the more it will be dissolved. More importantly for this chapter, the more nonpolar a compound is, the less it will be dissolved and the more it will tend to be a suspended particle.
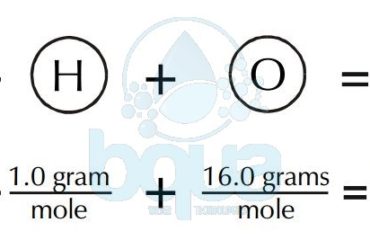
SIZE: One other aspect of suspended solids is their size. Very large molecules, even if they are polar, will tend to be particles. For example, we have discussed silica (SiO2) at length. If we look at the bonding between Si and O, we see that silicon has an electronegativity of 1.90 and O has an electronegativity of 3.44. Is this a polar bond? Given that water is polar and hydrogen has an electronegativity of 2.10, silica is more polar than water. Yet we see that sand is a particle and not dissolved.
To summarize, then, the degree of polarity and the size of an organic compound will determine if it is suspended or dissolved.