What is Electrodialysis ED
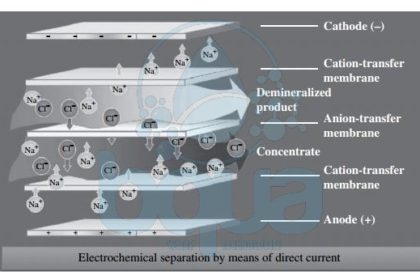
In electrodialysis (ED)–based desalination systems, the separation of minerals and product water is achieved through the application of direct electric current to the source water. This current drives the mineral ions and other ions with strong electric charge that are contained in the source water through ion-selective membranes to a pair of electrodes
of opposite charges. As ions accumulate on the surface of the electrodes, they cause fouling over time and have to be cleaned frequently in order to maintain a steady-state Electrodialysis ED process. A practical solution to this  challenge is to reverse the polarity of the oppositely charged electrodes periodically (typically two to four times per hour) in order to avoid frequent electrode cleaning.
An Electrodialysis ED process that includes periodic change of the polarity of the system’s electrodes is referred to as an electrodialysis reversal EDR process. At present, practically all commercially available ED systems are of the EDR type. Electrodialysis ED systems consist of a large number (300 to 600 pairs) of cation and anion exchange membranes separated by dilute flow dividers (spacers) to keep them from sticking together and to convey the desalinated flow through and out of the membranes. Each pair of membranes is separated from the adjacent pairs above and below it by concentrate spacers which collect, convey, and evacuate the salt ions retained between the adjacent membranes. The membranes used for ED are different from those applied for Reverse Osmosis RO desalination—they have a porous structure similar to that of microfiltration and ultrafiltration membranes. Reverse Osmosis membranes do not have physical pores. Electrodialysis ED membranes are more resistant to chlorine and fouling and are significantly thicker than RO membranes.
electrodialysis ED membrane desalination technology schematic process system
It is important to note that a single set of EDR stacks can only remove approximately 50 percent of salts. As a result, multiple EDR stacks connected in series are often used to meet more stringent product water Total Dissolved Solids TDS targets. It should be pointed out that compared to brackish water RO membranes, which typically yield only up to 85 to 90 percent recovery, Electrodialysis Reversal EDR systems can reach freshwater recovery of 95 percent or more. The energy needed for ED desalination is proportional to the amount of salt removed from the source water. TDS concentration and source water quality determine to a great extent which of the two membrane separation technologies (RO or ED) is more suitable and cost effective for a given application. Typically, ED membrane separation is found to be cost competitive for source waters with TDS concentrations lower than 3000 mg/L. This applicability threshold, however, is a function of the unit cost of electricity and may vary from project to project.
The TDS removal efficiency of Electrodialysis ED desalination systems is not affected by non-ionized compounds or objects with a weak ion charge (i.e., solids particles, organics, and microorganisms). Therefore, ED membrane desalination processes can treat source waters of higher turbidity and biofouling and scaling potential than can RO systems. However, the TDS removal efficiency of ED systems is typically lower than that of Reverse Osmosis systems (15.0 to 90.0 percent versus 99.0 to 99.8 percent), which is one of the key reasons why they have found practical use mainly for brackish water desalination. In general, Electrodialysis Reversal EDR systems can only effectively remove particles that have a strong electric charge, such as mono- and bivalent salt ions, silica, nitrates, and radium. EDR systems have a very low removal efficiency with regard to low-charged compounds and particles—i.e., organics and pathogens. Table below provides a comparison of the removal efficiencies of distillation, ED, and RO systems for key source water quality compounds.
| Contaminant | Distillation (%) | ED/EDR (%) | RO (%) |
| TDS | >99.9 | 50-90 | 90-99.5 |
| Pesticides, Organics/VOCs | 50-90 | <5 | 5-50 |
| Pathogens | >99 | <5 | >99.99 |
| TOC | >95 | <20 | 95-98 |
| Radiological | >99 | 50-90 | 90-99 |
| Nitrate | >99 | 60-69 | 90-94 |
| Calcium | >99 | 45-50 | 95-97 |
| Magnesium | >99 | 55-62 | 95-97 |
| Bicarbonate | >99 | 45-57 | 95-97 |
| Potassium | >99 | 55-58 | 90-92 |
One important observation from this table is that, as compared to distillation and RO separation, ED desalination only partially removes nutrients from the source water. This fact explains why EDR is often considered more attractive than RO or thermal desalination (which remove practically all minerals from the source water) if the planned use of the desalinated water is for agricultural purposes—i.e., generating fresh or reclaimed water for irrigation of agricultural crops.
Construction and equipment costs for brackish water reverse osmosis (BWRO) and EDR systems of the same freshwater production capacity are usually comparable, or EDR is less costly, depending on the Reverse Osmosis membrane fouling capacity of the source water. However, since the amount of electricity consumed by EDR systems is directly proportional to the source water’s salinity, at salinities of 2000 to 3000 mg/L the energy use of EDR systems usually exceeds that of BWRO or nanofiltration systems for source waters. Therefore, EDR systems are not as commonly used as RO systems for BWRO desalination and are never applied for seawater reverse osmosis (SWRO) desalination.
It should be pointed out, however, that salinity is not the only criterion for evaluating the cost competitiveness of EDR and BWRO systems. Often, other compounds such as silica play a key role in the decision making process. For example, at the largest operational EDR plant worldwide at present—the 200,000 m3/day Barcelona desalination facility in Spain—this technology was preferred to BWRO desalination because the brackish surface water source for this plant—the Llobregat River—contains very high level of silica, which would limit recovery from a BWRO plant to only 65 percent; the EDR system can achieve 90 percent recovery. In addition, the Llobregat River was found to have very high organic content, which was projected to cause heavy fouling and operational constraints on a BWRO plant of similar size.
Reference: “Desalination Engineering” by Nikolay Voutchkov