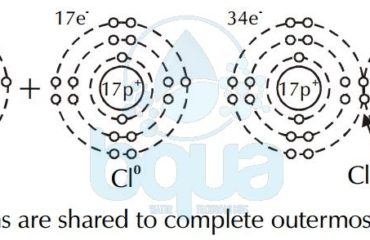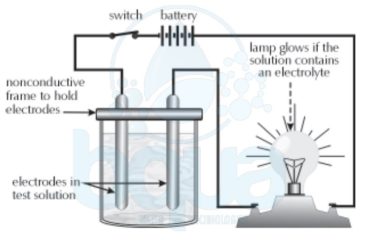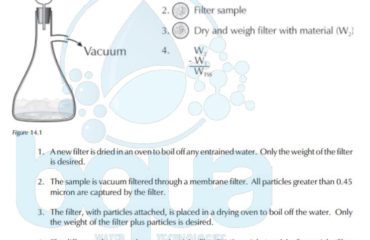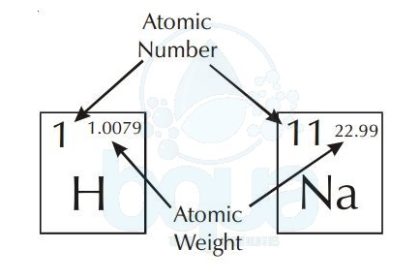
What is Atomic Weight
The atomic weight is simply the weight of one mole (6.022×10^23 atoms) of an element. The periodic table of elements shows the atomic weight on the upper right. The picture below shows an example of Hydrogen and Sodium atoms. Atomic weight of Hydrogen is 1.0079 when rounded to the nearest tenth = 1.0 gram. Same with Sodium atom, atomic weight is equal to 23.0 grams. For a molecule, to calculate the molecular weight we add the sum of atomic weights of the atoms in molecule.
atomic number and atomic weight in periodic table of elements
On the other hand, a gram atomic weight is the weight of one mole of an element, equal to its weight expressed in grams.