What is a molecule?
A molecule is a compound that was a substance formed by the chemical bonding of elements. Like an atom is the smallest particle of an element, the smallest discrete particle of a compound is a molecule.
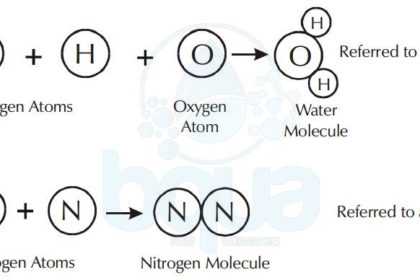
A molecule is made up of atoms from one or more elements. For example, the water molecule consists of two atoms of hydrogen chemically bonded to one oxygen atom. The nitrogen molecule consists of two nitrogen atoms bonded together.
Water Compound formed from Hydrogen Atoms and Oxygen Atoms
One mole equals 6.022×10^23 atoms (602,200,000,000,000,000,000,000 atoms). It is also called Avogadro Number or Avogadro Constant for the man credited for the number. Remember, one mole, or one atomic weight, or one molecular weight, or one Avogadro’s Number of any substance always contains 6.022×10^23 atoms, or molecules of that substance.
In the case of atoms, we describe the weight of one mole; the gram atomic weight. In the case of molecules, we describe it as the gram molecular weight.
Molecules are described by chemical formulas which use the chemical symbols found on the periodic table. Along with numbers to describe the number of atoms of that particular element found in the molecule.
Molecule Name Composition:
– CaSO4 – Gypsum, Calcium Sulfate – One atom of calcium, one sulfur, and four of oxygen.
– NaHCO3 – Baking Soda, Sodium Bicarbonate – One atom of sodium, one atom of hydrogen, one atom of carbon, and three atoms of oxygen.
– Al2(SO4)3 – Alum, Aluminum Sulfate – Two atoms of aluminum, three atoms of sulfur, and twelve oxygen.
– H2S – “Rotten Egg Gas”, Hydrogen Sulfide – Two hydrogens, and one atom of sulfur.