What is a mole definition
Atoms are very small. In order to find a unit to measure an atom; scientists decided to use a unit consisting of a large group of atoms while quantifying them. The unit used for atomic measurement is called a mole.
A particular number of atoms of each element is equal to a mole of this element. A unit of measurement of the weight of a mole of atoms is called the gram atomic weight of the element and it is expressed in grams. On the other hand, one mole of a specific molecule is a gram molecular weight of this molecule.
You can find the atomic weight number in the upper right hand corner of the element box on the periodic table of elements. It basically expresses the weight of one mole of this particular element in grams.
For example, one mole of the hydrogen atom weighs 1.0079 grams. Since numbers are generally rounded off to the nearest tenth of a gram, one mole of hydrogen atoms weighs 1.0 gram. While a weight of one mole of Carbon is 12.0 grams.
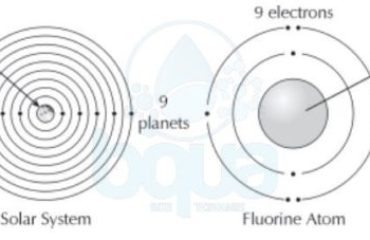
atomic measurement what is a mole definition avogadro number
Avogadro Number is one mole
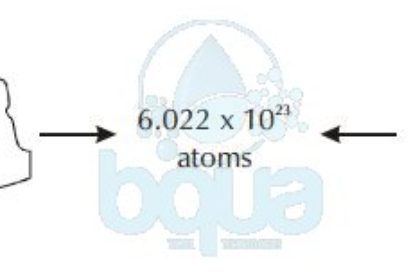
A mole is equal to 6.022×10^23 atoms (602,200,000,000,000,000,000,000 atoms). It is also called Avogadro Number for the man who first acknowledged the number.
One mole, or one atomic weight, or one molecular weight, or one Avogadro’s Number of any substance always contains 6.022 x 10^23 atoms, or molecules of that substance, not necessarily atoms.