What is a Reverse Osmosis System – RO System Definition
A Reverse Osmosis System is basically the application of the reverse of the Osmosis process. Where pure water is produced out of brackish or seawater by applying a pressure to the concentrated salt solution above the applied and osmotic back pressures. An Industrial Reverse Osmosis System works the same way as illustrated. Net driving pressure (NDP) forces water through the membrane. In an operating Reverse Osmosis system, feed water is pressurized by a high pressure pump. Due to Net Driving Pressure (NDP), a portion of the feed water is forced through the Reverse Osmosis semipermeable membrane.
The membrane is completely impermeable (won’t allow passage) to particles and only slightly permeable to dissolved substances. The water that passes through the membrane is called permeate. Permeate usually has very few particles in it. Unless there is a membrane defect (hole) or other problem, any particles found in the permeate were produced there (either from bacteria or equipment). Permeate is also low in dissolved substances (a small amount of dissolved solids does pass through the membrane). Permeate, therefore, is a relatively high purity water. Figure below illustrates a Reverse Osmosis System in the format we have been using so far.
Reverse Osmosis System (RO System) Illustration
Reverse Osmosis System Operation and Flushing
We know that when we pressurize the feed water and water passes through the membrane, the feed water is concentrated. If the concentration in the feed water gets high enough, the osmotic back pressure will rise to eventually give us a Net Driving Pressure (NDP) of zero and the flow will stop. In a Reverse Osmosis System, then, we must flush away the dissolved substances from the membrane surface so that the osmotic back pressure won’t keep going up. This is different from the other filters that we usually work with. Most of the filters that we have dealt with in our lives have been “full flowâ€, “accumulative†types of filters. “Full flow†means that there is one stream in (feed water) and one stream out (filtrate). “Accumulative†means that the filtered “stuff†accumulate in or on the filter.
From coffee filters, to cartridge filters, to multimedia filters, this has been the case. The feed water goes in, the filter removes the “stuff†that we want to take. When the filter gets full, we backwash or replace the filter. Membrane systems can’t be full flow or accumulative. With an RO membrane, we are filtering out ions which have an osmotic pressure. What would happen if we continue to filter out dissolved substances which produce an osmotic back pressure? Answer: The process would stop.
A Reverse Osmosis System therefore, must have a flushing flow which carries away the dissolved and suspended substances. This waste stream is called concentrate. A Reverse Osmosis System (RO System), therefore, has one stream going into it (Feed Water), and two streams coming out (Permeate & Concentrate).
The following illustration also shows a Reverse Osmosis System with a 100 gpm (22.7 m3/hr) feed water flow. The Net Driving Pressure (NDP) supplied by a high pressure pump forces around 75% of the feed water through the membrane. The water, suspended particles, and dissolved substances which don’t go through the membrane are concentrated and exit the Reverse Osmosis System as concentrate.
Reverse Osmosis System Operation Example
Reverse Osmosis System Contaminants Removal
Most water constituents retains on the feed side of the Reverse Osmosis membrane depending on their size and electric charge. While the purified water (permeate) passes through the membrane. Figure below illustrates the sizes and types of solids removed by Reverse Osmosis membranes as compared to other commonly used filtration technologies. RO membranes can reject particulate and dissolved solids of practically any size. However, they do not reject well gases, because of their small molecular size. Usually Reverse Osmosis membranes remove over 90 percent of compounds of 200 daltons (Da) or more. One Da is equal to 1.666054 × 10−24 g. In terms of physical size, RO membranes can reject well solids larger than 1 (Angstrom) Å. This means that they can remove practically all suspended solids, protozoa (i.e., Giardia and Cryptosporidium), bacteria, viruses, and other human pathogens contained in the source water. Reverse Osmosis membranes are designed to primarily reject soluble compounds (mineral ions) while retaining both particulate and dissolved solids.
BQUA Reverse Osmosis RO Membrane Contaminant Removal
The structure and configuration of Reverse Osmosis membranes is such that they cannot store and remove from their surface large amounts of suspended solids. If left in the source water, the solid particulates would accumulate and quickly plug (foul) the surface of the Reverse Osmosis membranes. Not allowing the membranes to maintain a continuous steady-state desalination process. Therefore, the suspended solids (particulates) in desalination feed water must be removed before reaching the RO membranes.
Over the past 20 years, RO membrane separation has evolved more rapidly than any other desalination technology. Mainly because of its competitive energy consumption and water production costs. Brackish Water Reverse Osmosis System (BWRO) yields the lowest overall production costs of all the desalination technologies. It is also important to note that the latest Multi-Effect Distillation MED projects built over the last 5 years have been completed at costs comparable to those for similarly sized Seawater Reverse Osmosis plants. Seawater Reverse Osmosis (SWRO) desalination – for the majority of medium and large projects – is usually is more cost competitive than thermal desalination technologies.
Example of an Industrial Brackish Water Reverse Osmosis System BWRO
- Published in Technology, Water Treatment
What is Reverse Osmosis (RO) Definition
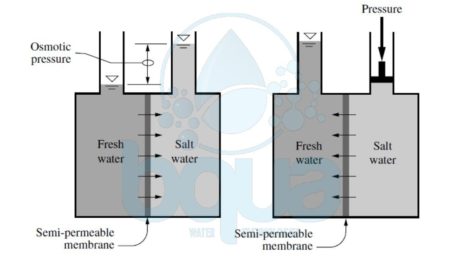
Reverse osmosis (RO) is basially the reverse of the osmosis process. Scientists found that all that is required to reverse the process of osmosis is a suitable semipermeable membrane and applying a pressure to the concentrated salt solution above the applied and osmotic back-pressures, thereby forcing pure water through the semipermeable membrane. In other words, reverse osmosis is the process where water containing inorganic salts (minerals), suspended solids, soluble and insoluble organics, aquatic microorganisms, and dissolved gases (collectively called source water constituents or contaminants) is forced under pressure through a semipermeable membrane. Semipermeable refers to a membrane that selectively allows water to pass through it at much higher rate than the transfer rate of any constituents contained in the water. Learn more about pressure driven membranes here. If water of high salinity is separated from water of low salinity via a semipermeable membrane, a natural process of transfer of water will occur from the low-salinity side to the high-salinity side of the membrane until the salinity on both sides reaches the same concentration. This natural process of water transfer through a membrane driven by the salinity gradient occurs in every living cell; it is known as osmosis.
The hydraulic pressure applied on the membrane by the water during its transfer from the low-salinity side of the membrane to the high-salinity side is termed osmotic pressure. Osmotic pressure is a natural force similar to gravity and is proportional to the difference in concentration of total dissolved solids (TDS) on both sides of the membrane, the source water temperature, and the types of ions that form the TDS content of the source water. This pressure is independent of the type of membrane itself. In order to remove fresh (low-salinity) water from a high-salinity source water using membrane separation, the natural osmosis-driven movement of water must be reversed, i.e., the freshwater has to be transferred from the high-salinity side of the membrane to the low-salinity side. For this reversal of the natural direction of freshwater flow to occur, the high-salinity source water must be pressurized at a level higher than the naturally occurring osmotic pressure.
If the high-salinity source water is continuously pressurized at a level higher than the osmotic pressure and the pressure losses for water transfer through the membrane, a steady-state flow of freshwater from the high-salinity side of the membrane to the low-salinity side will occur, resulting in a process of salt rejection and accumulation on one side of the membrane and freshwater production on the other. This process of forced movement of water through a membrane in the opposite direction to the osmotic force driven by the salinity gradient is known as reverse osmosis (RO).
The rate of water transport through the membrane is several orders of magnitude higher than the rate of passage of salts. This significant difference between water and salt passage rates allows membrane systems to produce freshwater of very low mineral content. The applied feed water pressure counters the osmotic pressure and overcomes the pressure losses that occur when the water travels through the membrane, thereby keeping the freshwater on the low-salinity (permeate) side of the membrane until this water exits the membrane vessel.
Osmosis and Reverse Osmosis Process
The salts contained on the source water (influent) side of the membrane are retained and concentrated; they are ultimately evacuated from the membrane vessel for disposal. As a result, the RO process results in two streams—one of freshwater of low salinity (permeate) and one of feed source water of elevated salinity (concentrate, brine or retentate), as shown in the figure above. While semipermeable RO membranes reject all suspended solids, they are not an absolute barrier to dissolved solids (minerals and organics alike). Some passage of dissolved solids will accompany the passage of freshwater through the membrane. The rates of water and salt passage are the two key performance characteristics of Reverse Osmosis membranes.
Reverse Osmosis Process Drawing
- Published in Technology, Water Treatment