What is a Covalent Bond
Atoms can bond by sharing electrons in their outermost orbitals and thus giving them the privilege of having a full outermost orbital. For example, the element Hydrogen exists as H2 molecule consisting of two atoms of Hydrogen atom. A covalent bond is simply the force between two atoms resulting from the sharing of electrons in the outermost orbital. Therefore a covalent bond attracts two atoms very close to each others because both atoms share the same electrons in their outermost orbitals.
hydrogen gas formed by covalent bond sharing electron between two hydrogen atoms
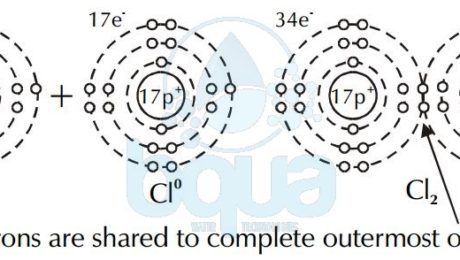
The case of Hydrogen cannot be an ionic bond. That is because Hydrogen ions each have the same charge and therefore are not attracted to one another. This is why a covalent bond happens only when two atoms have a difference in electronegativity of > or = 0.9. Instead, electrons are shared between the atoms to fill the outermost orbital. This type of bond is called a covalent bond. Two atoms of chlorine (Cl0) share a pair of electrons to form chlorine gas (Cl2).
electron shared covalent bond chlorine atom forming chlorine gas
Some molecules formed by covalent bonds may still give up or acquire one or more electrons resulting in a net positive or negative charge. These molecules are called molecular ions.
A covalent bond is very strong. The energy needed to break it is bigger than the thermal energy existing at 25°C which is room temperature. The thermal energy at 25°C is <1 kCal/mole kilo calorie per mole, while to break a typical Carbon covalent bond in an Ethane molecule we need about 83 Kilocalorie per mole.
Properties of molecules defined by covalent bond
While a central atom in a molecule attracts other atoms by covalent bonds, the bonds between these atoms are forming particular angles between them. The force of repulsion among these atoms and particularly the outermost electrons is what shape and determine the angles degrees. The angles and the shape formed is what gives the molecule its properties and shape. An example is the angle formed between Oxygen and Hydrogen atoms in a water molecule, an angle of 105°. You can read about the molecular geometry.
When atoms formed in a covalent bond have the same or very close electronegativity – typically less than 0.9 – the bond (the force of attraction) is equal between electrons since they’re almost identical. This kind of bond is called a non-polar bond. Carbon-Carbon and Carbon-Hydrogen bonds (also called Hydrocarbon) is an example on non-polar bonds.
A polar bond occurs when the difference in electronegativity between two atoms are more than or equal to 0.9. In a polar bond, there’s a partial negative charge (δ−) and a partial positive charge (δ+). This is the case in a water molecule H2O. The bond between Oxygen and Hydrogen is polar since difference in electronegativity is 1.2. (Electronegativity of Oxygen is 3.4 and Electronegativity of Hydrogen is 2.2).
- Published in Water Chemistry, Water Treatment
What is Surface Tension
Surface tension is one of the properties of water that is created by the Hydrogen bond. Surface tension is the reason a water droplet can have different shapes on different surfaces. As we all noticed, when we fill a glass of water to the top, water level may actually be above the glass rim. The explanation for this occurrence is the high surface tension of water on a hydrophilic surface like glass due to hydrogen bond.
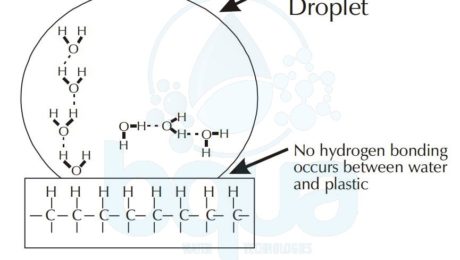
surface tension of water on plastic hydrophobic
surface tension of water droplet on hydrophilic glass
Hydrophobic and Hydrophilic Examples
On the other hand, a water droplet tends to form a sphere like shape on a hydrophobic surface like for example plastic. Therefore, a drop of water will tend to spread on a clean glass surface. To simply explain why this happens, we will analyse the phenomenon on a molecular level. In the example of plastic and since it is a nonpolar substance; water will tend form hydrogen bonds with itself. In the example of glass which is polar; water molecules will form hydrogen bonds with the silicon dioxide of the glass. And this is what makes water tend to spread on the glass surface; attraction to glass. Read more about polarity.
Another example explaining surface tension, using a small glass tube called a capillary tube. If we place the end tip of this tube in a glass of water, water will drawn. This is because of the hydrogen bond occurring between the SiO2 of the glass tube and the Oxygen atoms in the water molecules. The definition of hydrophilic which is what describes the glass in this situation means water loving. While definition for Hydrophobic which is plastic in the above example means water hating.
surface tension capillary tube example water glass hydrophilic
A unit of measurement for the surface tension of water is dynes/cm at room temperature. Which is the force you have to overcome in order to break the surface tension of a water droplet (1cm in length). The lower the temperature the less surface tension. Hot water makes a better cleaning solution since it is considered a better wetting agent. This is due to low surface tension of hot water which allows it to better react with detergents which are hydrocarbons with a polar end called a functional group.
- Published in Water Chemistry, Water Treatment
What is a Hydrogen Bond
A water molecule will form a bond called a hydrogen bond. A hydrogen bond is formed because of the polarity of the molecule and the fact that hydrogen atoms are present in the molecule. water molecules will form hydrogen bond with another molecule(s) of water. These bonds are not nearly as strong as the covalent bond between the hydrogen atoms and the oxygen atom within one H2O molecule.
We can simply say that a Hydrogen Bond is a weak bond that occurs between a proton of a molecule and an electronegative atom of another molecule as a result of an electrostatic attraction between the two molecules. The Hydrogen bond can either happen between two different molecules or within the same molecule.
Hydrogen bond gives water many of its amazing properties, including:
– High Boiling Point
–Â Surface Tension
– Capillary Action
– Solvent Ability
The molecular weight of water is 18; which is the sum of the atomic weights of the atoms in one molecule. Most compounds of such a small molecular weight (MW) are gases at room temperature and pressure.
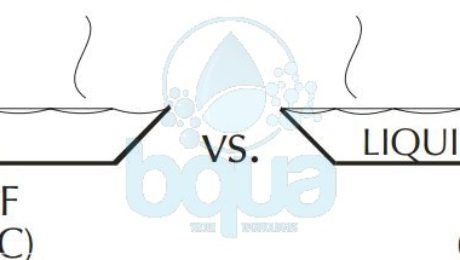
Considering the example of nitrogen (N2) as compared to water. Nitrogen has a MW of 28 and a boiling point of 321°F (-196°C) below zero. Water, on the other hand, has a MW of 18 and a boiling point of 212°F (100°C), a difference of 533°F (296°C).
hydrogen bond water and nitrogen difference properties
The difference between nitrogen and water is that the nitrogen molecule has no force of attraction between molecules. Water, of course, has the hydrogen bond between molecules. Although the hydrogen bonds are weak compared to other bonds, they are significant enough to create the tremendous difference in boiling points between the two compounds. The picture below shows few molecules of water and molecules of nitrogen within a pure liquid state.
hydrogen bonding water nitrogen difference in properties
- Published in Water Chemistry, Water Treatment