What is an ion
An Ion is simply an atom with a charge. In order to explain what is an ion, we will briefly review the atom structure. Electron configuration teaches us that an atom is more stable when its outermost orbital has a whole set of electrons. Actually, the tendency of an atom to achieve a complete outer most orbital is very powerful that it will overcome the electrostatic force that hold the atom together.
Like the case of the Fluorine atom, it will accept an extra electron to achieve a full outmost orbital even
though it will end up having  one more electrons than protons. Basically, a neutral atom has the same number of protons and electrons.
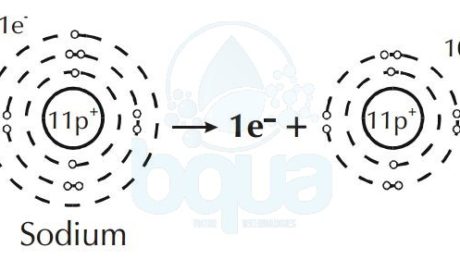
Furthermore, an element like Sodium will give up an electron to attain a full outer orbital. This results in a difference and unbalance between electrons and protons in the same atom.
When in an atom protons (+ve) are more than electrons (-ve) = result is positive ion (cation).
Sodium atom loses one electron become Sodium positive ion cation
Some atoms give up more than one electron to have a total outermost orbital. While other atoms will acquire more than one electron in order to fill the outer orbital. Since electrons have a negative charge (negative ion) and protons have a positive charge (positive ion), a difference in the number of electrons and protons results a net charge. This is when an atom is therefore called an ion.
Depending upon whether it gains or loses an electron, an ion will have either a positive or a negative
charge. Cations are ions with a net positive charged and anions are ions with a net negative charge. We know that opposite charges are attracted while same charges are repelled. That means that cations and anions are attracted to one another. The strength of the attraction may make ions form a solid called a crystal lattice.
- Published in Water Chemistry, Water Treatment
What is a Functional Group
Molecular compounds owe their individual properties to functional group. These are combinations of atoms which may appear in various parts of the molecule. A list of major functional group listed below.
| Illustrative Example | Name of Functional Class | Functional Group |
| CH3CH2CH3 | Alkanes | C-C |
| CH3CH=CH2 | Alkenes | C=C |
| CH3CH/CH | Alkynes | C/C |
| CH3CH2CH2OH | Alcohols | C–OH |
| Aldehydes | ||
| Ketones | ||
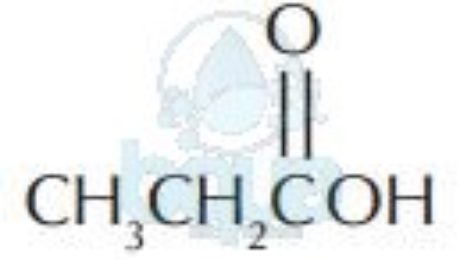
| Acids | ||
| CH3CH2CH2NH2 | Amines | C-NH2 |
| CH3OCH2CH3 | Ethers | -C-O-C- |
| CH3CH2CH2Br | Halides | C-Br |
The difference in electronegativity of two bonding atoms determines if the bond will be polar or nonpolar (read about polarity). The bond between two identical atoms is always nonpolar, (for example: C-C, C=C, C=C; H-H, O=O, etc…). Bonds between atoms with electronegativities which are nearly the same are nonpolar (for example C-H). Bonds between atoms with electronegativities which are significantly different (commonly 0.9) are polar (for example, C-O, H-O, C=O, and N-H).
One molecule, especially a large complex molecule, may have more than one type of functional group. One specific functional group may be repeated several times.
A functional group may alter the properties of a compound such as melting point, boiling point, solid structure, and solubility. A functional group on an organic molecule also contribute to its hydrophobic and hydrophilic properties. Surfaces composed of nonpolar molecules will be hydrophobic. Surfaces composed of compounds containing polar bonds will be hydrophilic. You can also read about surface tension.
- Published in Water Chemistry, Water Treatment
What is Surface Tension
Surface tension is one of the properties of water that is created by the Hydrogen bond. Surface tension is the reason a water droplet can have different shapes on different surfaces. As we all noticed, when we fill a glass of water to the top, water level may actually be above the glass rim. The explanation for this occurrence is the high surface tension of water on a hydrophilic surface like glass due to hydrogen bond.
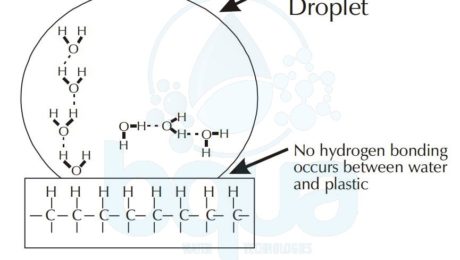
surface tension of water on plastic hydrophobic
surface tension of water droplet on hydrophilic glass
Hydrophobic and Hydrophilic Examples
On the other hand, a water droplet tends to form a sphere like shape on a hydrophobic surface like for example plastic. Therefore, a drop of water will tend to spread on a clean glass surface. To simply explain why this happens, we will analyse the phenomenon on a molecular level. In the example of plastic and since it is a nonpolar substance; water will tend form hydrogen bonds with itself. In the example of glass which is polar; water molecules will form hydrogen bonds with the silicon dioxide of the glass. And this is what makes water tend to spread on the glass surface; attraction to glass. Read more about polarity.
Another example explaining surface tension, using a small glass tube called a capillary tube. If we place the end tip of this tube in a glass of water, water will drawn. This is because of the hydrogen bond occurring between the SiO2 of the glass tube and the Oxygen atoms in the water molecules. The definition of hydrophilic which is what describes the glass in this situation means water loving. While definition for Hydrophobic which is plastic in the above example means water hating.
surface tension capillary tube example water glass hydrophilic
A unit of measurement for the surface tension of water is dynes/cm at room temperature. Which is the force you have to overcome in order to break the surface tension of a water droplet (1cm in length). The lower the temperature the less surface tension. Hot water makes a better cleaning solution since it is considered a better wetting agent. This is due to low surface tension of hot water which allows it to better react with detergents which are hydrocarbons with a polar end called a functional group.
- Published in Water Chemistry, Water Treatment
What is an Atom Definition
An atom is the most discrete indivisible particle that forms an element. Atoms of the same element are identical; different elements have non-similar atoms. The most simple of all elements is Hydrogen.Â
Example: Hydrogen atoms are similar while Oxygen atoms are different than Hydrogen atoms. Two hydrogen atoms and one Oxygen combined form the water molecule H2O.
The atoms are extremely small. Therefore, even the most powerful microscopes cannot see them. Actually, 6.022X10^23 hydrogen atoms (Avogadro Number) weigh only one gram. There are 454 grams in one pound.
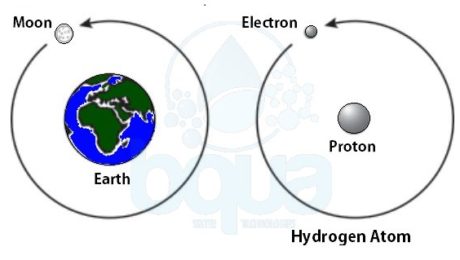
In order to understand the nature of the atom, we should identify the forces that are holding it together. Much as our plant, gravity is the force holding the moon in its orbit around the earth. Gravity is the force of attraction between two large masses.
In the case of the atom, the small size of the particles and the speed at which the electron travels would prohibit a force such as gravity from being able to hold the atom together. Scientists have learned that electrostatic force is the force that maintains the atom.
Atoms Composition
While atoms are composed of small subatomic particles; these particles are the electron, the proton and the neutron. The proton and neutron are found in the nucleus which is the center of the atom. The electron is negatively charged and orbits around the nucleus.
atom composed of subatomic particles electron proton neutron
Atoms from different elements are constituted of the same type of subatomic particles. However, the proportions of the subatomic particles are different for each element. Hydrogen for example has two subatomic particles; an electron and a proton. In the case of hydrogen, a single electron orbits a single proton. ÙAn example is the moon which orbits the earth. Same as in the case of earth, the proton of the hydrogen atom is much larger than the electron.
an electron orbits proton in nucleus in an atom like moon orbits earth
Moreover, if we add a subatomic particle to a Hydrogen atom, we create another atom of another element. Note: The number of subatomic particles is what characterizes atoms of different elements. In addition, if we want to create a Helium atom, we should only add an additional electron, an additional proton and two neutrons to the Hydrogen atom. As a result, we’ll have two electrons orbiting four other particles, two protons and two neutrons in the nucleus.
- Published in Water Chemistry, Water Treatment