What is Cadmium – Cadmium Element Definition
Cadmium enters the environment from a variety of industrial applications, including mining and smelting operations, electroplating, and battery, pigment, and plasticizer production. Cadmium element occurs as an impurity in zinc and may also enter consumers’ tap water by galvanized pipe corrosion. Cadmium is also in food, with 27 ug/day in the average diet.
Cadmium element acts as an emetic and can cause kidney dysfunction, hypertension, anemia, and altered liver function. It can build up in the kidney with time. Chronic occupational exposure has resulted in renal dysfunction and neuropsychological impairments. The mechanisms remain uncertain, but cadmium element competes with calcium inside cells and across cell membranes. Cadmium has been shown to induce testicular and prostate tumors in laboratory animals injected subcutaneously. Lung tumor incidence is increased in people exposed to cadmium by inhalation.
As yet, USEPA considers cadmium element unclassifiable as a human carcinogen regarding oral exposure because the observed human carcinogenicity occurs via inhalation. It is regulated based on its renal toxicity in humans, and, using an uncertainty factor of 10, an MCLG and an MCL of 5 ug/L have been adopted (U.S. Environmental Protection Agency, 1991e).
- Published in Water Chemistry, Water Treatment
What is Asbestos – Asbestos Element Definition
Asbestos is the name for a group of naturally occurring, hydrated silicate minerals with fibrous appearance. Included in this group are the mineral chrysotile, crocidolite, anthophyllite, and some of the tremolite-actinolite and cummingtonite-grunerite series. All except chrysotile fibers are known as amphibole. Most commercially mined asbestos is chrysotile. Asbestos element occurs in water exposed to natural deposits of these minerals, asbestos mining discharges, and asbestos-cement pipe.
The physical dimensions of asbestos fibers rather than the type are more important in health effects, with the shorter, thinner fibers more highly associated with cancer by inhalation. Human occupational and laboratory animal inhalation exposures are associated with the cancer, mesothelioma, found in lung, pleura, and peritoneum. An NTP study also observed gastrointestinal cancers in rats dietarily exposed to intermediate range fiber (65 percent of the fiber larger than 10 um, 14 percent larger than 100 μm) for their lifetime. Epidemiologic studies of asbestos element in drinking water have had inconsistent results, but there are suggestions of elevated risk for gastric, kidney, and pancreatic cancers (Cantor, 1997). The USEPA based its 1/1,000,000 cancer risk estimate and the MCLG and MCL on 7 × 106 fibers/L > 10 micron observed in the NTP rat study (USEPA).
- Published in Water Chemistry, Water Treatment
What is Arsenic – Arsenic Element Definition
Arsenic concentrations in U.S. drinking waters are typically low. However, an estimated 5,000 community systems (out of 70,000) using groundwater and 370 systems (out of 6,000) using surface water were above 5 ug/L. These were primarily in the western states. Dissolution of arsenic-containing rocks and the smelting of nonferrous metal ores, especially copper, account for most of the arsenic in water supplies. Until the 1950s, arsenic element was also a major agricultural insecticide.
Arsenic element may be a trace dietary requirement and is present in many foods such as meat, fish, poultry, grain, and cereals. Market-basket surveys suggest that the daily adult intake of arsenic is about 50 ug, with about half coming from fish and shellfish. In fish, fruit, and vegetables, it is present in organic arsenical forms, which are less toxic than inorganic arsenic. However, arsenic is not currently considered essential (National Research Council, 1989). Extrapolating from animal studies, Uthus (1994) calculated a safe daily intake of between 12 and 40 ug.
In excessive amounts, arsenic element causes acute gastrointestinal damage and cardiac damage. Chronic doses can cause Blackfoot disease, a peripheral vascular disorder affecting the skin, resulting in the discoloration, cracking, and ulceration. Changes in peripheral nerve conduction have also been observed. Epidemiological studies in Chile, Argentina, Japan, and Taiwan have linked arsenic in drinking water with skin, bladder, and lung cancer (reviewed by Smith et al., 1992; Cantor, 1997). Some studies have also found increased kidney and liver cancer.
Ingestion of arsenical medicines and other arsenic exposures have also been associated with several internal cancers, but several small studies of communities in the United States with high arsenic levels have failed to demonstrate any health effects. Micronuclei in bladder cells are increased among those chronically ingesting arsenic in drinking water. Inorganic arsenate and arsenite forms have been shown to be mutagenic or genotoxic in several bacterial and mammalian cell test systems and have shown teratogenic potential in several mammalian species, but cancers have not been induced in laboratory animals.
USEPA has classified arsenic as a human carcinogen, based primarily on skin cancer (U.S. Environmental Protection Agency, 1985).The ability of arsenic to cause internal cancers is still controversial. Under the NIPDWR regulations, an MCL of 50 ug/L had been set, but it is under review. Currently, USEPA’s Risk Assessment Council estimates that an RfD (for non-carcinogenic skin problems) ranges from 0.1 to 0.8 ug/kg/day−1, which translates into an MCLG of 0 to 23 ug/L. Based on a 1-in-10,000 risk of skin cancer, USEPA estimated that 2 ug/L might be an acceptable limit for arsenic in drinking water.
- Published in Water Chemistry, Water Treatment
What is Aluminum – Definition
Aluminum element occurs naturally in nearly all foods, the average dietary intake being about 20 mg/day. Aluminum salts are widely used in antiperspirants, soaps, cosmetics, and food additives. Aluminum is common in both raw and treated drinking waters, especially those treated with alum. It is estimated that drinking water typically represents only a small fraction of total aluminum intake. Aluminum element shows low acute toxicity, but administered to certain laboratory animals is a neurotoxicant. Chronic high-level exposure data are limited, but indicate that aluminum affects phosphorus absorption, resulting in weakness, bone pain, and anorexia.
Carcinogenicity, mutagenicity, and teratogenicity tests have all been negative. Associations between aluminum and two neurological disorders, Alzheimer’s disease and dementia associated with kidney dialysis, have been studied. Current evidence suggests that Alzheimer disease is not related to aluminum intake from drinking water, but other sources of aluminum appeared to be associated with Alzheimer. Dialysis dementia has been reasonably documented to be caused by aluminum. Most kidney dialysis machines now use specially prepared water.
Aluminum element was included on the original list of 83 contaminants to be regulated under the 1986 SDWA amendments. USEPA removed aluminum from the list because it was concluded that no evidence existed at that time that aluminum ingested in drinking water poses a health threat (U.S. Environmental Protection Agency, 1988b). USEPA has a secondary maximum contaminant level (SMCL) of 50 to 200 μg/L to ensure removal of coagulated material before treated water enters the distribution system.
- Published in Water Chemistry, Water Treatment
What is Electronegativity – Electronegativity Definition
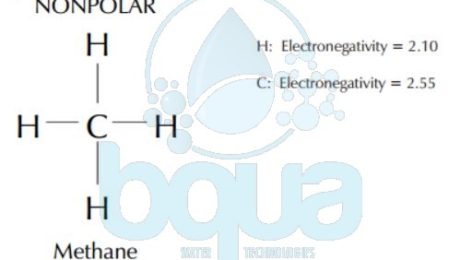
The electronegativity determines whether a bond between two atoms is polar or nonpolar which is defined by Polarity. The electronegativity is a measurement of how attractive an atom is to electrons. The more electronegative an element is, the more the shared electrons will spend around that atom. When one atom in a bond gets to keep the electrons more than the other, it assumes a partial negative charge. The other bonded atom has the electrons less often, so assumes a slight positive charge. Like the negative and positive poles of a magnet. One end of the bond is positive and one end is negative, so the bond is said to be polar. Carbon and hydrogen bonds, as in methane, are nonpolar because the electronegativity of both are very close. Carbon and oxygen bonds are polar because the electronegativity of them are substantially different.
Electronegativity example in Methane molecule
Polar substances dissolve in polar substances because the partial positive & negative charges of one molecule are attracted to the partial +ve and -ve charges of other molecules. Nonpolar substances will dissolve in nonpolar substances. This is because of other forces of attraction, such as Van der Waals’ forces, can come into play. Nonpolar compounds generally do not dissolve in polar compounds, and vice versa, because there is no attraction between things that are charged and things that aren’t. Since water is polar, compounds which are polar will generally dissolve well.
Therefore, if we have an organic which is polar, such as methyl alcohol, it will dissolve well in water. In general, the more polar an organic is, the more it will be dissolved. More importantly for this chapter, the more nonpolar a compound is, the less it will be dissolved and the more it will tend to be a suspended particle.
SIZE: One other aspect of suspended solids is their size. Very large molecules, even if they are polar, will tend to be particles. For example, we have discussed silica (SiO2) at length. If we look at the bonding between Si and O, we see that silicon has an electronegativity of 1.90 and O has an electronegativity of 3.44. Is this a polar bond? Given that water is polar and hydrogen has an electronegativity of 2.10, silica is more polar than water. Yet we see that sand is a particle and not dissolved.
To summarize, then, the degree of polarity and the size of an organic compound will determine if it is suspended or dissolved.
- Published in Water Chemistry, Water Treatment
What is Total Suspended Solids – TSS Definition
Total suspended solids (TSS) concentration is a measure of the total weight of solid residuals contained in the source water. It is customarily presented in milligrams per liter or parts per million. Total Suspended Solids can also be defined as a laboratory analysis that measures the weight of suspended solids in a specified volume. It is used to accurately (within the limitations of the test) measure suspended solids concentration. The TSS measurement is the standard method for measuring the weight of suspended solids. The method utilizes a 0.45 micron membrane filter.
Total Suspended Solids TSS measurement test procedure
Total Suspended Solids is the most accurate standard test for monitoring the amount of suspended material in a sample. The problems with it are:
1. It doesn’t tell us anything about the number of particles.
2. It doesn’t tell us anything about the size of particles.
3. It is only a crude measurement of the fouling potential of the particles.
4. It doesn’t measure any particles less than 0.45 micron in diameter.
Even with its limitations, however, it can be a valuable tool for monitoring the solids loading into a Reverse Osmosis System.
TSS is measured by filtering a known volume of water (typically 1 L) through a preweighed glass-fiber filter. Drying the filter with the solids retained on it at 103 to 105°C, and then weighing the filter again after drying. The difference between the weight of the dried filter and of the clean filter, divided by the volume of the filtered sample. Reflects the total amount of particulate (suspended) solids in the source water.
It should be pointed out that because saline water contains dissolved solids which will crystallize and convert into particulate solids when the sample is heated at 103 to 105°C. Often Total Suspended Solids TSS analysis of saline water completed in accordance with the standard methods. For water and wastewater analysis yields an erroneously high Total Suspended Solids TSS content in the water. The higher the source water’s salinity and the lower its particulate content. The more inaccurate this measurement is. In order to address the challenge associated with the standard method of Total Suspended Solids TSS measurement. It is recommended to wash the solids retained on the filter by spraying the filter with deionized water before drying. Unless the source water solids sample is washed before drying, the results of this sample are meaningless.
The laboratory Total Suspended Solids TSS test is completed properly and the filtered sample is well washed to give us a precise measure. This parameter usually provides a much more accurate measure of the actual content of particulate solids in the source water than does turbidity. Because it accounts for the actual weight of the particulate material present in the sample. For comparison, turbidity measurement is dependent on particle size, shape, and color. And typically is not reflective of particles of very small size (i.e., particles of 0.5 µm or less). Such as fine silt and picoalgae. In fact, a change in the ratio of Total Suspended Solids TSS to turbidity is a good indicator of a shift in the size of particles contained in the source water. Which may be triggered by algal blooms, storms, strong winds, and other similar events. Which can result in resuspension of solids from the bottom sediments into the water column.
Typically, an increase in the Total Suspended Solids/turbidity ratio is indicative of a shift of particulate solids toward smaller-size particles. For example, during non-algal-bloom conditions, the TSS/turbidity ratio of an appropriately processed sample is typically in the range of 1.5 to 2.5. Water with a turbidity of 2 NTU would have a Total Suspended Solids concentration of 3 to 5 mg/L. During heavy algal blooms dominated by small-size (pico- and micro-) algae, the TSS of the source water could increase over 10 times (e.g., to 40 mg/L). While the source water turbidity could be multiplied by 2 to 3 times only. For this example it would be in range of 4 to 6 NTU. With a corresponding increase in the TSS/NTU ratio from 2/1 to between 6/1 and 10/1.
- Published in Water Chemistry, Water Treatment
What is Coagulation Definition
Coagulation is simply the process that destabilizes colloidal particles so that they can come together to form larger, conglomerate particles. Low-pressure membrane technology is becoming significantly more prevalent in the drinking water industry. Low-pressure membranes are purely size-exclusionary devices. As a result, anything smaller than membrane pore sizes (approximately 0.01 to 0.1 micron) will pass through the membrane, Therefore, membrane feed waters with dissolved materials, such as organics and metals, require some form of additional treatment.
Often, in these cases, the most economical pre-treatment process is simple coagulation. Potential coagulants for membrane pre-treatment include those also used for conventional water treatment. Additionally, organic adsorption media such as PAC and MIEX, or oxidants such as potassium permanganate, chlorine, or chlorine dioxide can be applied upstream of a low-pressure membrane (assuming appropriate membrane compatibility) for enhanced dissolved material removal.
Similar to a direct filtration mode of operation for conventional technology, the goal of coagulation for membrane pretreatment is to produce a pinpoint floc that is capable of adsorbing dissolved matter, but minimizes solids loading onto the membrane filtration process. As noted briefly above, it is important to quantify membrane compatibility and performance with the coagulant of choice.
Each commercially available RO membrane utilizes different membrane materials. As a result, the compatibility and performance of a coagulant for membrane filtration pre-treatment will likely vary between membrane system and raw water supplies. As such, there are no specific guidelines for membrane system pre coagulation except the general guidelines that are associated with conventional treatment.
Coagulant Types
The coagulant most frequently used for membrane plant source seawater conditioning prior to sedimentation or filtration is ferric salt (ferric sulfate and ferric chloride). Aluminum salts (such as alum or polyaluminum chloride) are not typically used because it is difficult to maintain aluminum concentrations at low levels in dissolved form because aluminum solubility is very pH dependent. Small amounts of aluminum may cause mineral fouling of the downstream Seawater Reverse Osmosis membrane elements. In coagulation, coagulant dosage for a given source water should be determined based on jar test and/or pilot testing. The optimum coagulant dosage in a coagulation process is pH dependent and should be established based on an on-site jar or pilot testing for the site-specific conditions of a given application.
Overdosing of a coagulant used for seawater pretreatment is one of the most frequent causes for SWRO membrane mineral fouling. When overdosed during coagulation, coagulant accumulates on the downstream facilities and can cause fast-rate fouling of downstream cartridge filters following the pretreatment step and in iron fouling of the Seawater RO membranes. The effect of overdosing of coagulant (iron salt) on the SDI level can be recognized by visually inspecting the SDI test filter paper (Figure 6). In such situation, a significant improvement of source water SDI can be attained by reducing coagulant feed dosage or in case of poor mixing, modifying the coagulant mixing system to eliminate the content of unreacted chemical in the filtered seawater fed to the SWRO membrane system.
In line static mixer used in coagulation
The main purpose of the coagulation system is to achieve uniform mixing of the added coagulant with the source seawater and efficient coagulation of the particles contained the seawater. The two types of mixing systems most widely used in seawater desalination plant is in line static mixer and mechanical flash mixer installed in coagulation tanks. Although in-line mixers are simple and less costly, they have two disadvantages: (1) their mixing efficiency is a function of the flow rate; (2) static mixers are proprietary equipment and the project designer would need to rely on the equipment manufacturer for performance projections.
Static mixers also create additional head losses of 0.5 to 1.0 meters, which need to be accounted for in the design of the intake pump station. Another important issue is to provide adequate length of pipeline (at least 20 times the pipe diameter) between the mixer and the entrance to the pretreatment filters in order to achieve adequate flocculation. Mechanical flash mixing systems consist of coagulation tank with one or more mechanical mixers and chambers. The coagulation tank is designed for a mixing time (t) of 1 to 3 seconds and mechanical mixers that create velocity gradient (G) of 300 s, (optimum G x t = 500 to 1,600). The power requirement for the mechanical mixer is 2.2 to 2.5 horsepower/10,000 m3/day. This type of mixing usually provides a more reliable and consistent coagulation, especially for desalination plants with significant daily flow variations (i.e., more than 30 % of average annual production flow).
- Published in Water Chemistry, Water Treatment
What is Total Organic Carbon – TOC Definition
Total organic carbon is one of the most widely used measures for organic content of seawater. Total Organic Carbon concentration measures the content of both Natural Organic Matter (NOM) and of easily biodegradable organics, such as polysaccharides released during algal blooms. This water quality parameter is widely used, because it is relatively easy to measure. And it is indicative of the tendency of the seawater to cause Seawater Reverse Osmosis System SWRO membrane biofouling. Total Organic Carbon TOC is measured by converting organic carbon to carbon dioxide in high-temperature furnace in the presence of a catalyst.
Typically, open ocean seawater which is not influenced by surface fresh water influx (nearby river confluence). By man-made activities (i.e., wastewater or storm water discharges, or ship traffic). Or by algal bloom event (i.e., red tide), has a very low Total Organic Carbon content ($0.2 mg/L). When an algal bloom occurs however, Total Organic Carbon TOC concentration of the ocean water could increase by an order of magnitude (2 to 8 mg/L). Similar magnitude of Total Organic Carbon (TOC) increase could be triggered by a storm water or river discharge during high-intensity rain event. Such as the rainy seasons in tropical and equatorial parts of the world.
Usually, an increase of Total Organic Carbon above a certain threshold (2.0 to 2.5 mg/L) is observed to trigger accelerated biofouling of Seawater RO membranes. The Carlsbad seawater desalination demonstration plant in California is supplied by seawater collected using near-shore open ocean intake. Observations indicate that Total Organic Carbon concentration in the source water at that location exceeds 2.0 mg/L during algal bloom events. Within a week to two week period, the Seawater Reverse Osmosis system experiences measurable biofouling and associated increase in operating pressure. Similar TOC level observations at the Tampa seawater desalination plant, in Florida, USA (where the typical background TOC level of the seawater is less than 4 mg/L) indicate that accelerated biofouling occurs when Total Organic Carbon concentration exceeds 6 to 8 mg/L.
Usually, accelerated biofouling at the Tampa facility is triggered by one of two key events – rain events, which increase the content of alluvial organics in the source seawater, or algal blooms which cause elevated organic levels due to massive die off of algae. The increase in alluvial organics during rain events is caused by the elevated flow and alluvial content of Alafia River. Which discharges into Tampa Bay several kilometers upstream of the desalination plant intake. During high-intensity rains during summer months, TOC level in the river water discharging in the bay may exceed 20 mg/L.
| Seawater Source | TOC (mg/l) | Polysaccharides (% of Total TOC) |
Humic Substances and Building Blocks (% of Total TOC) |
Low Molecular Weight Acids and Neutrals (% of Total TOC) |
Other Low Molecular Weight Compounds (% of Total TOC) |
| Surface Raw Seawater – Perth, Australia | 0.9 | 3 | 31 | 25 | 41 |
| Surface Raw Seawater -Ashkelon, Israel May 2005) |
1.2 | 14 | 39 | 25 | 22 |
| Surface Raw Seawater -Ashkelon, Israel May 2006) |
1 | 7 | 52 | 22 | 19 |
| Surface Raw Seawater -Carboneras, Spain | 0.9 | 8 | 38 | 18 | 42 |
| Well Seawater – Gibraltar, Spain | 0.6 | 1 | 26 | 22 | 51 |
| Surface Raw Seawater –Gibraltar, Spain | 0.8 | 5 | 26 | 25 | 42 |
Analysis of various sources of seawater indicates that TOC concentration of seawater may contain various fractions of organics. This depends on the origin of this water and the type of seawater intake. See Table above. These fractions may also change depending on the season as well. Analysis of the Table indicates that low-molecular weight organic compounds are typically the greatest fraction of the TOC in seawater (40 to 50%). Comparison of the data from the Ashkelon seawater desalination plant in Israel indicates that the most easily biodegradable organics (polysaccharides) change seasonally. And increase during the summer season along with the content of algal biomass in the ocean. This data also shows that TOC concentration of seawater may not always correlate with the content of polysaccharides in the water.
- Published in Water Chemistry, Water Treatment
Que es un ion
Un ion es simplemente un atomo con una carga. Para explicar que es un ion, revisaremos brevemente la estructura del atomo. La configuracion electronica nos ensena que un atomo es mas estable cuando su orbital mas externo tiene un conjunto completo de electrones. En realidad, la tendencia de un atomo a lograr un orbitario externo mas completo es muy poderosa y superara la fuerza electrostatica que mantiene unido al atomo.
Al igual que en el caso del atomo de fluor, aceptara un electron adicional para lograr un orbital mas externo, a pesar de que terminara teniendo uno mas electrones que protones. Basicamente, un atomo neutro tiene el mismo numero de protones y electrones.
Ademas, un elemento como el sodio abandonara un electron para alcanzar un orbital externo completo. Esto resulta en una diferencia y desequilibrio entre electrones y protones en el mismo atomo.
Cuando en un atomo, los protones (+ve) son mas que los electrones (-ve) = el resultado es un ion positivo (cation).
Algunos atomos abandonan mas de un electron para tener un orbital mas externo. Mientras que otros atomos adquiriran mas de un electron para llenar el orbital externo. Dado que los electrones tienen una carga negativa (ion negativo) y los protones tienen una carga positiva (ion positivo), una diferencia en el numero de electrones y protones da como resultado una carga neta. Esto es cuando un átomo se llama por lo tanto un ion.
Dependiendo de si gana o pierde un electron, un ion tendra una carga positiva o negativa. El catión es un ion que tiene una carga positiva neta y el anión es un ion que tiene una carga negativa neta. Sabemos que los cargos opuestos se atraen, mientras que los mismos cargos son rechazados. Eso significa que los cationes y los aniones se atraen entre si. La fuerza de la atraccion puede hacer que los iones formen un solido llamado retÃculo de cristal.
- Published in Water Chemistry
Que es la geometria molecular?
La geometria molecular es basicamente la disposicion tridimensional / forma / estructura de los atomos que forman una molecula. Cuando las moleculas estan formadas por un enlace quimico, lo que significa que los atomos se unen entre si, los suborbitales involucrados en el enlace o enlaces crean diferentes formas moleculares que dependen de muchos factores.
Por ejemplo, las moléculas de agua no son lineales, una molecula de agua en realidad tiene forma de “V” y el angulo formado entre los dos atomos de hidrogeno y el atomo de oxÃgeno es aproximadamente de 105 grados.
Cuando dibujamos moleculas en dos dimensiones, la mayoria de las veces pensamos que estas moleculas son planas. Pero en realidad existen en diferentes formas y formas.
La Composicion Quimica y la Geometria Molecular de una molecula es lo que determina principalmente las propiedades de la molecula. Como el sabor, el punto de ebullicion, el magnetismo, la dinamica, la polaridad, el color y todas las demas propiedades.
Geometria molecular y diferentes tipos de estructuras moleculares:
Geometria Molecular Lineal
En una estructura de Geometria Molecular Lineal, los atomos estan unidos entre si para formar una lÃnea recta. Los angulos de union son de 180 grados y, por ejemplo, el dioxido de carbono (CO2) y el oxido nitrico (NO).
Planar Triangular – Geometria Molecular Planar Trigonal
Trigonal Planar Molecular La geometria se forma cuando un compuesto tiene un atomo en el centro unido a otros tres atomos en una disposicion que parece un triangulo alrededor del atomo central. Los cuatro atomos estan en la misma linea y planos en un plano.
Geometria Molecular Piramidal Trigonal
La Geometria Molecular Piramidal Trigonal obviamente tiene la forma de una piramide con una base que parece un triangulo. La estructura piramidal trigonal se parece a la geometria molecular tetraedrica, las estructuras piramidales necesitan tres dimensiones para que puedan separar completamente los electrones. Un ejemplo de la orientacion piramidal trigonal es el amoniaco (NH3).
Geometria Molecular Tetraedrica
Tetra significa cuatro y tetraedrico significa basicamente un solido o piramide que tiene cuatro lados. La geometria molecular tetraedrica se forma cuando un atomo central tiene cuatro enlaces con cuatro atomos a la vez formando una forma de piramide con cuatro lados. Segun la VSEPR, que es la teoria de la repulsion del par de electrones de la carcasa de valencia, los ángulos de enlace entre los atomos en la orientacion tetraedrica son aproximadamente 109.47 grados. Un ejemplo de la Geometria Molecular Tetraedrica es el metano (CH4).
Geometria Molecular Plana Cuadrada
La geometria molecular plana y cuadrada se forma cuando un atomo central tiene cuatro enlaces y dos pares solitarios. El tetrafluoruro de xenon (XeF4) es un ejemplo de la estructura plana cuadrada; está formado por seis orbitales equidistantes dispuestos en ángulos de 90 grados. Que forma una forma octaedrica. Dos orbitales contienen pares de electrones solitarios en lados opuestos del atomo central. Y los otros cuatro atomos estan unidos al atomo central, lo que hace que la molecula tenga una estructura plana y cuadrada.
Geometria Molecular Bipiramidal Trigonal
La Geometria Molecular Bipiramidal Trigonal ocurre cuando el atomo central esta conectado a cinco atomos formando cinco enlaces y sin pares solitarios. Tres de los cinco enlaces se crean a lo largo del ecuador del atomo formando angulos de 120 grados mientras que los dos restantes se forman en el eje del atomo. Un ejemplo de la Geometria Molecular Bipiramidal Trigonal es el Pentacloruro de Fósforo (PCl5).
Geometria Molecular Octaedrica
Octa significa ocho y Geometria Molecular Octaedrica significa una piramide o solido que tiene ocho lados o caras. La estructura octaedrica tiene seis atomos unidos que forman angulos de 90 entre ellos. Un ejemplo de la estructura molecular octaedrica es el hexafluoruro de azufre (SF6).
Formas moleculares y diferentes tipos de estructuras moleculares.
- Published in Water Chemistry
What is Conductivity Definition
Water conductivity is a measurement of dissolved ions. Water conductivity indirectly measures ions by measuring the passage of electrons through a sample of water. Solutions which have a lot of dissolved ions will have a high conductivity. Solutions which have a low concentration of dissolved ions will have a low conductivity.
Water Conductivity: Pure Water vs Seawater
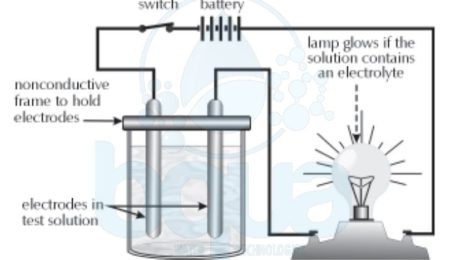
Conductance in a solution actually involves the movement of ions. Picture shows an apparatus which can be used to show that solutions of dissolved ions (dissolved salts) in water will pass the electric potential of a battery through them and allow the light bulb to light. Electrolyte definition is simply a solution which will conduct a current.
Water Conductivity Measurement Using Apparatus
Positively-charged ions are called “cationsâ€, negatively-charged ions are called “anions.†Cations are formed when one or more electrons are lost from an atom or a group of atoms, anions are formed when one or more electrons are gained by an atom or group of atoms. An electrolyte contains cations and anions. The more cations & anions present, the higher the conductivity. If the amount of current passing through a solution is measured with a conductivity meter, we can tell relatively how many ions are dissolved in the solution. When more current passes through the solution, we know more ions are present.
Water Conductivity decreases as temperature decreases, whereas the conductance of a solid conductor (such as a copper wire) increases with decreasing temperature. Water Conductance in would therefore appear to be different than in a solid, and it is. Water Conductivity actually occurs as a result of positive and negative ions moving through the solution. This is illustrated in the picture. Table salt, sodium chloride (NaCl), has been dissolved in water to create an electrolyte, and an ammeter has been installed to measure current.
Water Conductivity Measurement Using Salt Ammeter
In the electrolyte shown above, the electric potential is actually being passed through the liquid by the movement of cations and anions. The cations (sodium ions) are attracted to the negative electrode and migrate to it. The anions (chloride ions) are attracted to the positive electrode then migrate to it. Once a negative chloride ion reaches the positive pole, it donates an electron, and once a positive sodium ion reaches the negative pole it gains an electron. The transferred electron (chloride to sodium) passes through the ammeter and registers as current (conductivity).
It is important to note that water conductivity does not tell us what type of ions are present. Conductivity is only a relative measurement of the total number of ions present. Also, some ions exhibit a higher conductivity than others because they are more mobile. Hydrogen ions are the most conductive ions. Hydroxide ions are the next most conductive. All other ions fall below these two as far as conductivity is concerned.
For example, a solution of calcium sulfate may give the same conductivity as a solution of NaCl although the calcium sulfate solution may have more total ions than the sodium chloride solution. This is because sodium and chloride are smaller ions and travel through the solution much more rapidly.
Pure water will also exhibit some, although very little, conductivity. This is due to the fact that water will ionize each of the hydrogen ion and hydroxide (OH) ions very slightly. Recall that water ionizes to the extent of 1 x 10^-7 moles per liter, producing 1×10^-7 moles/liter of hydrogen ions and same number of mol/l of hydroxide ions.
H2O ——-> [H+] + [OH-]
- Published in Water Chemistry, Water Treatment
ما هو الرقم الهيدروجيني – مقياس الأس الهيدروجيني
تعتبر Øموضة وقلوية المØلول مهمة للغاية ÙÙŠ معالجة المياه Ø¨Ø§Ù„ØªÙ†Ø§Ø¶Ø Ø§Ù„Ø¹ÙƒØ³ÙŠ بسبب عوامل مثل تدهور الغشاء ØŒ وتنظي٠الأغشية ØŒ وما إلى ذلك. وذلك لأن بعض التÙاعلات الكيميائية ستØدث Ùقط عند مقياس أس هيدروجيني Ù…Øددة.
يستخدم Ø§Ù„Ù…ØµØ·Ù„Ø Ø§Ù„Ø£Ø³ الهيدروجيني لوص٠ما إذا كان المØلول قلوي أم Øامضي. Ø£Ùضل وص٠لمÙهوم الØموضة والقلوية هو العودة إلى ثابت التÙكك.
بالنظر إلى الأس الهيدروجيني Øول تركيز الهيدروجين H + ion ØŒ نرى أنه مع ارتÙاع تركيز H + (ويقل تركيز OH) Ù†Øصل على رقم سلبي أصغر. وبالمثل ØŒ مع ارتÙاع تركيز الهيدروكسيد (ويقل تركيز H +) Ù†Øصل على رقم سلبي أكبر.
[H +] x [OH-] = 1.0 x 10 ^ -14
مع إضاÙØ© الØمض (H +)
1.0 x 10 ^ -5 x 1.0 x 10 ^ -9 = 1.0 x 10 ^ -14
مع إضاÙØ© القاعدة (OH-)
1.0 x 10 ^ -9 x 1.0 x 10 ^ -5 = 1.0 x 10 ^ -14
تغييرات الأس الهيدروجيني مع التغيرات ÙÙŠ H + وتركيزات OH
مع أخذ ذلك ÙÙŠ الاعتبار ØŒ علم العلماء أن طريقة أكثر ملاءمة لوص٠تركيز أيون الهيدروجين ÙÙŠ المØلول هو أخذ اللوغاريتم السالب لتركيز أيونات الهيدروجين. اللوغاريثم هو الأس الهيدروجيني الذي يتم رÙع رقم أساس منه لإنتاج رقم معين. ملاØظة: سنستخدم عادة قاعدة من 10.
مثال: السجل (اللوغاريتم) من 100 هو 2: عشرة مرÙوع إلى قوة 2 (102).
مثال: سجل 127 هو 2.1 (على الآلة الØاسبة العلمية ØŒ أدخل 127 ØŒ ثم اضغط المÙØªØ§Ø [LOG]).
| رقم | لوغاريتم | رقم | لوغاريتم |
| 1 = 1 X 10^0 | 0 | 1.0 = 1 X 10^0 | 0 |
| 10 = 1 X 10^1 | 1 | 0.1 = 1 X 10^-1 | -1 |
| 100 = 1 X 10^2 | 2 | 0.01 = 1 X 10^-2 | -2 |
| 1000 = 1 X 10^3 | 3 | 0.001 = 1 X 10^-3 | -3 |
| 10000 = 1 X 10^4 | 4 | 0.0001 = 1 X 10^-4 | -4 |
| 100000 = 1 X 10^5 | 5 | 0.00001 = 1 X 10^-5 | -5 |
| 1000000 = 1 X 10^6 | 6 | 0.000001 = 1 X 10^-6 | -6 |
| 10000000 = 1 X 10^7 | 7 | 0.0000001 = 1 X 10^-7 | -7 |
رمز السجل السالب هو “p”. لذلك ØŒ Ùإن السجل السلبي لتركيز أيون الهيدروجين هو “مقياس الأس الهيدروجيني”. يسرد الجدول أدناه العديد من تركيزات أيونات الهيدروجين وتركيزات هيدروكسيد ودرجة الØموضة المقابلة Ùˆ pOH. لاØظ أن الرقم الهيدروجيني 7 Ù…Øايدة لأن تركيز أيون الهيدروجين [H +] وتركيز الهيدروكسيد [OH-] هما Ù†Ùس الشيء. كما يزيد [H +] ØŒ ينقص الأس الهيدروجيني.
| H+ (mol/L) | OH- (mol/L) | ||||
| عدد عشري | SN | pH | عدد عشري | SN | pOH |
| 0.00000000000001 | 1 X 10^-14 | 14 | 1.0 | 1 X 10^0 | 0 |
| 0.0000000000001 | 1 X 10^-13 | 13 | 0.1 | 1 X 10^-1 | 1 |
| 0.000000000001 | 1 X 10^-12 | 12 | 0.01 | 1 X 10^-2 | 2 |
| 0.00000000001 | 1 X 10^-11 | 11 | 0.001 | 1 X 10^-3 | 3 |
| 0.0000000001 | 1 X 10^-10 | 10 | 0.0001 | 1 X 10^-4 | 4 |
| 0.000000001 | 1 X 10^-9 | 9 | 0.00001 | 1 X 10^-5 | 5 |
| 0.00000001 | 1 X 10^-8 | 8 | 0.000001 | 1 X 10^-6 | 6 |
| 0.0000001 | 1 X 10^-7 | 7 | 0.0000001 | 1 X 10^-7 | 7 |
| 0.000001 | 1 X 10^-6 | 6 | 0.00000001 | 1 X 10^-8 | 8 |
| 0.00001 | 1 X 10^-5 | 5 | 0.000000001 | 1 X 10^-9 | 9 |
| 0.0001 | 1 X 10^-4 | 4 | 0.0000000001 | 1 X 10^-10 | 10 |
| 0.001 | 1 X 10^-3 | 3 | 0.00000000001 | 1 X 10^-11 | 11 |
| 0.01 | 1 X 10^-2 | 2 | 0.000000000001 | 1 X 10^-12 | 12 |
| 0.1 | 1 X 10^-1 | 1 | 0.0000000000001 | 1 X 10^-13 | 13 |
| 1.0 | 1 X 10^-0 | 0 | 0.00000000000001 | 1 X 10^-14 | 14 |
- Published in Water Chemistry, Water Treatment
What is pH – pH Scale Definition
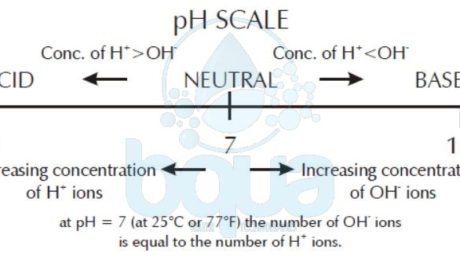
pH refers to the concentration of hydrogen ions in solution. The lower the pH the more hydrogen
ions present. The higher the pH the fewer hydrogen ions present
pH scale: Concentration of Hydrogen ions
The acidity and alkalinity of a solution is extremely important in Reverse Osmosis water treatment due to factors such as membrane degradation, membrane cleaning, etc. This is because certain chemical reactions will only take place at specific pH values.
The term pH is used to describe whether a solution is alkaline or acidic. The concept of acidity and alkalinity may best be described by going back to the dissociation constant.
Looking at the exponent on the concentration of Hydrogen H+ ion, we see that as the H+ concentration goes up (and OH- concentration goes down) we get a smaller negative number. Likewise, as Hydroxide OH- concentration goes up (and H+ concentration goes down) we get a larger negative number.
[H+] x [OH-] = 1.0 x 10^-14
With addition of acid (H+)
1.0 x 10^-5 x 1.0 x 10^-9 = 1.0 x 10^-14
With addition of base (OH-)
1.0 x 10^-9 x 1.0 x 10^-5 = 1.0 x 10^-14
Exponent changes with changes in H+ and OH- concentrations
Taking this into consideration, scientists learned that a more convenient way to describe the hydrogen ion concentration of a solution is to take the negative logarithm of the hydrogen ion concentration. A logarithm is the exponent to which a base number is raised to produce a given number. NOTE: We will usually use a base of 10.
EXAMPLE: The log (logarithm) of 100 is 2: Ten raised to the power of 2 (102).
EXAMPLE: The log of 127 is 2.1 (On a scientific calculator, enter 127, then push the [LOG] key).
| NUMBER | LOG | NUMBER | LOG |
| 1 = 1 X 10^0 | 0 | 1.0 = 1 X 10^0 | 0 |
| 10 = 1 X 10^1 | 1 | 0.1 = 1 X 10^-1 | -1 |
| 100 = 1 X 10^2 | 2 | 0.01 = 1 X 10^-2 | -2 |
| 1000 = 1 X 10^3 | 3 | 0.001 = 1 X 10^-3 | -3 |
| 10000 = 1 X 10^4 | 4 | 0.0001 = 1 X 10^-4 | -4 |
| 100000 = 1 X 10^5 | 5 | 0.00001 = 1 X 10^-5 | -5 |
| 1000000 = 1 X 10^6 | 6 | 0.000001 = 1 X 10^-6 | -6 |
| 10000000 = 1 X 10^7 | 7 | 0.0000001 = 1 X 10^-7 | -7 |
The symbol for the negative log is “p”. Therefore, the negative log of the Hydrogen ion concentration is “pH”. Table below lists several hydrogen ion concentrations, hydroxide concentrations, and the corresponding pH and pOH. Note that a pH of 7 is neutral because the Hydrogen ion concentration [H+] and hydroxide concentration [OH-] are the same. As [H+] increases, pH decreases.
| H+ (mol/L) | OH- (mol/L) | ||||
| Decimal | SN | pH | Decimal | SN | pOH |
| 0.00000000000001 | 1 X 10^-14 | 14 | 1.0 | 1 X 10^0 | 0 |
| 0.0000000000001 | 1 X 10^-13 | 13 | 0.1 | 1 X 10^-1 | 1 |
| 0.000000000001 | 1 X 10^-12 | 12 | 0.01 | 1 X 10^-2 | 2 |
| 0.00000000001 | 1 X 10^-11 | 11 | 0.001 | 1 X 10^-3 | 3 |
| 0.0000000001 | 1 X 10^-10 | 10 | 0.0001 | 1 X 10^-4 | 4 |
| 0.000000001 | 1 X 10^-9 | 9 | 0.00001 | 1 X 10^-5 | 5 |
| 0.00000001 | 1 X 10^-8 | 8 | 0.000001 | 1 X 10^-6 | 6 |
| 0.0000001 | 1 X 10^-7 | 7 | 0.0000001 | 1 X 10^-7 | 7 |
| 0.000001 | 1 X 10^-6 | 6 | 0.00000001 | 1 X 10^-8 | 8 |
| 0.00001 | 1 X 10^-5 | 5 | 0.000000001 | 1 X 10^-9 | 9 |
| 0.0001 | 1 X 10^-4 | 4 | 0.0000000001 | 1 X 10^-10 | 10 |
| 0.001 | 1 X 10^-3 | 3 | 0.00000000001 | 1 X 10^-11 | 11 |
| 0.01 | 1 X 10^-2 | 2 | 0.000000000001 | 1 X 10^-12 | 12 |
| 0.1 | 1 X 10^-1 | 1 | 0.0000000000001 | 1 X 10^-13 | 13 |
| 1.0 | 1 X 10^-0 | 0 | 0.00000000000001 | 1 X 10^-14 | 14 |
- Published in Water Chemistry, Water Treatment
What is Dissociation Constant Definition
Dissociation Constant demonstrates the maximum range to which an element or substance would dissociate into ions. The Dissociation Constant referred to as “K” is equal to the product of the concentrations of the corresponding ions:
K = [H+] x [OH-]
The dissociation constant for a compound such as sodium chloride is very large since the ions are almost totally dissociated (exist as separate cations and anions). Dissociation constants for compounds which do not readily dissociate (separate) are small.
Highly Soluble Salts <——-> Large Dissociation Constant
Slightly Soluble Salts <——> Small Dissociation Constant
Most ionic compounds will dissociate to some extent. Even water will slightly dissociate as described by the equation below.
H2O —-> (H+) + (OH-)
The dissociation constant for water is found by multiplying the concentrations of the hydrogen ion (H+) and the hydroxide ion (OH-). The brackets in the equation indicate that we are dealing with concentrations expressed in molarity. Scientists found that the product of concentration of the two ions is 1.0 x 10-14 at standard conditions.
K = [H+] x [OH-] = 1.0 x 10^-14
If we are dealing with pure water, we know that the concentration of H+ and OH- must be the same since one of each is required to make a water molecule.
[H+] = [OH-]
Since,
(H+) + (OH-) —–> H2O
Therefore, if the concentrations of the ions multiplied together are equal to 1.0 x 10^-14 and the concentrations of each ion are the same, we know that the concentration of each ion is 1.0 x 10^-7. Remember, when we multiply numbers with exponents, we add the exponents together.
[H+] x [OH-] = 1.0 x 10^-14
1.0 x 10^? x 1.0 x 10^? = 1.0 x 10^-14
[H+] and [OH-] are equal:
[H+] = [OH-] = 1.0 x 10^-14
1.0 x 10^-7 [H+] x 1.0 x 10^-7 [OH-] = 1.0 x 10^-14
If we add some hydrochloric acid (HCl) to the pure water, the concentration of hydrogen ions will increase since HCl is almost completely dissociated.
HCl + H2O —–> H+* + Cl- + H2O
*Concentration of H+ is increased by the addition of HCl
As the concentration of hydrogen ions increases, the concentration of hydroxide ions decreases. This is because the dissociation constant (product of the H+ concentration multiplied by the OH- concentration) for water does not change. It is a basic chemical characteristic just like density, boiling point, freezing point, etc.
[H+] x [OH-] = 1.0 x 10^-14
As the concentration of H+ goes up, the negative exponent on the concentration value goes down. In our example below, it goes from -7 to -5. The hydroxide exponent therefore must go from -7 to -9. Remember, the dissociation constant does not change. The product of the two concentrations (sum of the exponents) must always equal -14.
Example:
Pure Water
1.0 x 10^-7 x 1.0 x 10^-7 = 1.0 x 10^-14
With the addition of acid
1.0 x 10^-5* x 1.0 x 10^-9 = 1.0 x 10^-14**
*This number is larger due to the addition of acid.
**This number must remain the same.
In the same manner, if we add sodium hydroxide (NaOH) to pure water, the OH- concentration will increase. If the OH- concentration increases, the H+ concentration must decrease.
Now we can see that with water we can have one of three conditions. First, we can have a condition in which there are an equal number of H+ and OH- ions. Water in this state is said to be neutral. Second, we can have a condition in which we have more H+ than OH- ions. This condition is called acidic. Third, we can have a condition in which there are more OH- than H+ ions. This condition is called basic or alkaline.
[H+] > [OH-] —–> Acidic
[H+] = [OH-] —–> Neutral
[H+] < [OH-] —–> Alkaline
- Published in Water Chemistry, Water Treatment
What is Turbidity Definition
Turbidity is a parameter which measures the content of particulate foulants in the reverse osmosis feed water source. Turbidity can also be defined as the measurement of the light scattering properties of particles in suspension. The instrument used to measure turbidity is called a turbidimeter or turbidity meter.
The principle of turbidity meters is that suspended particles will block or reflect light. There are several different types of turbidity meters, but they are all based on the principle of particles blocking or reflecting light. Let’s look at reflected light first.
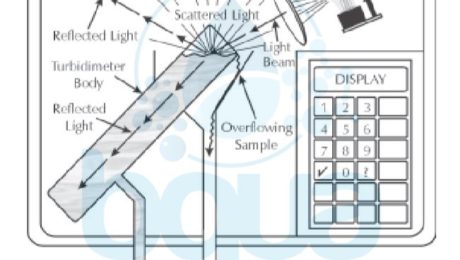
If there are no particles suspended in a sample, light will pass through the sample in a straight line. If a photocell (photoreceptor) is located at 90 degrees to the light path, no light will reach the photocell and the turbidity will read zero.
If particles present in a sample, the light will be reflected off of the surfaces of the particles and will be scattered at different angles. A photoreceptor at 90 degrees from the source of light will pick up light that is scattered and a turbidity measurement greater than 0 will be obtained.
The unit of measurement for turbidity is the NTU or the JTU or the FTU. There is no correlation between NTU and JTU and FTU. The term nephelometric indicates that this is a light-interference analytical technique. NTU is the measurement unit for light scattering. Some on-stream turbidity meters also use the light-scattering principle. A common one is called the surface scatter turbidimeter.
For a surface-scatter turbidimeter, the sample with suspended particles is passed up through a tube. A light source is shining on the surface of the liquid as it flows over the top of the tube. A photocell is located above the surface of the water. If there are no particulates in the water, the light beam is absorbed by the black tube and the black interior of the enclosure. If they are present, they scatter light as they surface (This is why this technique is called surface-scatter). The photocell picks up the scattered light and displays the reading in NTU.
There is another type of turbidimeter which doesn’t measure the light scattered but rather the reduction in transmitted light. In this case the full beam is received by the photocell. Full-beam reception equates to clean water and no turbidity.
 Â
If particles are present, less light reaches the photocell. This, then, is displayed as a turbidity reading, in JTU (Jackson Turbidity Unit, named after the man who created the standard candle which was originally used as the source of the light). Figure below illustrates particulates in the sample scattering, and therefore blocking, the light beams.
The turbidity level in the source of water is indicative of the content of clay, silt, suspended organic matter, and microscopic aquatic life. Such as phyto- and zooplankton. It is expressed in Nephelometric Turbidity Units (NTU) and Formazin Nephelometric Unit (FNU). The turbidity of open ocean and surface brackish water can vary between 0.1 and several hundred NTU, although under normal dry weather conditions, it is typically between 0.5 and 2.0 NTU. Rain events, algal blooms, storms, snow melt, river discharges, and human activity (such as wastewater discharges, ship traffic, etc.) can cause significant turbidity increases and variations. Usually, water that is saline with a turbidity below 0.05 NTU causes very low particulate fouling of the reverse osmosis membrane. Most RO membrane manufacturers have a maximum feed raw water turbidity of 1.0 NTU, although this level is relatively high in practical terms. Usually, filtered water turbidity below 0.1 NTU is desirable.
Although turbidity is a good measure of the overall content of particulates in the source water, on its own it is not an adequate parameter to characterize water’s potential for particulate or other fouling. Turbidity measurement does not provide information regarding the type and particle size in the source feed water and does not measure the constitution of dissolved organic and inorganic foulants. The size of particles contained in the source water matters because RO membrane feed and concentrate spacers, through which the saline raw water is distributed inside the membranes, are of limited width (typically 0.7 to 0.9 mm).
Even with these problems, turbidity measurements can be valuable trending indications for monitoring RO unit feed water. Most manufacturers require that the feed water to an RO unit be less than 1.0 NTU. Turbidity is almost always measured on feed waters which are processed through a clarifier. Clarifiers are usually found at facilities with a surface water source and no prior municipal treatment. In this case turbidity is frequently measured before and after the clarifier to monitor the performance of the clarifier. In a few cases, turbidity is measured before and/or after a multimedia filter to monitor performance of this piece of equipment. Turbidity or TSS measurements alone are insufficient to tell us about the fouling potential of our feed water. They must be used in conjunction with SDI (Silt Density Index).
- Published in Water Chemistry, Water Treatment
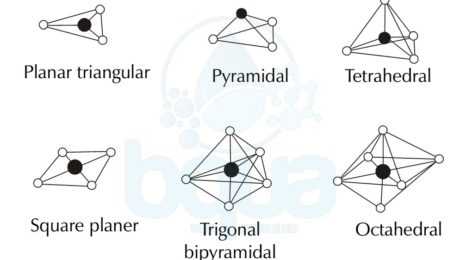
What is Molecular Geometry ?
Molecular Geometry is basically the three dimensional arrangement / shape / structure of atoms that form a molecule. When molecules are formed by chemical bond which means atoms bonding together, suborbitals involved in the bond or bonds create different molecular shapes depending on many factors.
For example, the water molecules are not linear, a water molecule is actually ‘V’ shaped and the angle formed between the two Hydrogen atoms and the Oxygen atom is approximately of 105° degrees.
molecular geometry of water molecule hydrogen oxygen
When we draw molecules in two dimensions, we most of the time think that these molecules are flat. But in fact they exist in several different shapes and forms.
The Chemical Composition and the Molecular Geometry of a molecule is what mainly determine the properties of the molecule. Such as taste, boiling point, magnetism, dynamic, polarity, color, and all other properties.
Molecular Geometry and different types of molecular structures:
-
Linear Molecular Geometry
In a Linear Molecular Geometry structure, atoms are bonded together to form a straight line. Bonding angles are of 180° degrees and as an example is the Carbon Dioxide (CO2) and the Nitric Oxide (NO).
-
Planar Triangular –Â Trigonal Planar Molecular Geometry
Trigonal Planar Molecualr Geometry is formed when a compound has an atom at the centre attached to three other atoms in an arrangement that looks like a triangle around the central atom. The four atoms are on the same line and flat on a plane.
-
Trigonal Pyramidal Molecular Geometry
Trigonal Pyramidal Molecular Geometry is obviously shaped like a pyramid with a base that looks like a triangle. The Trigonal Pyramidal structure looks like the Tetrahedral Molecular Geometry, pyramidal structures need three dimensions so that they can fully separate electrons. An example on the Trigonal Pyramidal orientation is Ammonia (NH3).
-
Tetrahedral Molecular Geometry
Tetra means four and tetrahedral means basically a solid or pyramid that has four sides. Tetrahedral Molecular Geometry is formed when one central atom has four bonds with four atoms all at once forming a pyramid-like shape with four sides. As per the VSEPR which is the Valence Shell Electron Pair Repulsion theory, the bond angles between the atoms in the tetrahedral orientation are approximately 109.47°. An example of the Tetrahedral Molecular Geometry is methane (CH4).
-
Square Planar Molecular Geometry
The Square Planar Molecular Geometry is formed when a central atom has four bonds and two lone pairs. Xenon Tetrafluoride (XeF4) is an example of the Square Planar structure, it is made up of six equally spaced orbitals arranged at 90° degrees angles. Which forms an octahedral shape. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. And the other four atoms are bonded to the central atom making the molecule a square planar structure.
-
Trigonal Bipyramidal Molecular Geometry
The Trigonal Bipyramidal Molecular Geometry happens when the central atom is connected to five atoms forming five bonds and no lone pairs. Three out of the five bonds are created along the atom equator forming 120° degrees angles while the remaining two are formed on the atom axis. An example on the Trigonal Bipyramidal Molecular Geometry is the Phosphorus Pentachloride (PCl5).
-
Octahedral Molecular Geometry
Octa means eight and Octahedral Molecular Geometry means a pyramid or solid that has eight sides or faces. Octahedral structure has six bonded atoms forming 90° degrees angles between them. An example on the Octahedral Molecular structure is Sulfur Hexafluoride (SF6).
Molecular Shapes and different types of molecular structures
- Published in Water Chemistry, Water Treatment
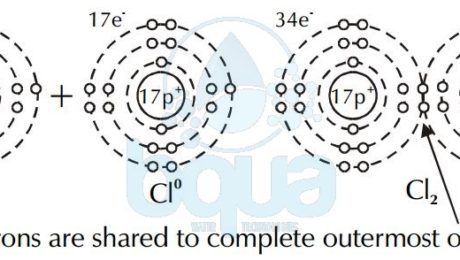
What is a Covalent Bond
Atoms can bond by sharing electrons in their outermost orbitals and thus giving them the privilege of having a full outermost orbital. For example, the element Hydrogen exists as H2 molecule consisting of two atoms of Hydrogen atom. A covalent bond is simply the force between two atoms resulting from the sharing of electrons in the outermost orbital. Therefore a covalent bond attracts two atoms very close to each others because both atoms share the same electrons in their outermost orbitals.
hydrogen gas formed by covalent bond sharing electron between two hydrogen atoms
The case of Hydrogen cannot be an ionic bond. That is because Hydrogen ions each have the same charge and therefore are not attracted to one another. This is why a covalent bond happens only when two atoms have a difference in electronegativity of > or = 0.9. Instead, electrons are shared between the atoms to fill the outermost orbital. This type of bond is called a covalent bond. Two atoms of chlorine (Cl0) share a pair of electrons to form chlorine gas (Cl2).
electron shared covalent bond chlorine atom forming chlorine gas
Some molecules formed by covalent bonds may still give up or acquire one or more electrons resulting in a net positive or negative charge. These molecules are called molecular ions.
A covalent bond is very strong. The energy needed to break it is bigger than the thermal energy existing at 25°C which is room temperature. The thermal energy at 25°C is <1 kCal/mole kilo calorie per mole, while to break a typical Carbon covalent bond in an Ethane molecule we need about 83 Kilocalorie per mole.
Properties of molecules defined by covalent bond
While a central atom in a molecule attracts other atoms by covalent bonds, the bonds between these atoms are forming particular angles between them. The force of repulsion among these atoms and particularly the outermost electrons is what shape and determine the angles degrees. The angles and the shape formed is what gives the molecule its properties and shape. An example is the angle formed between Oxygen and Hydrogen atoms in a water molecule, an angle of 105°. You can read about the molecular geometry.
When atoms formed in a covalent bond have the same or very close electronegativity – typically less than 0.9 – the bond (the force of attraction) is equal between electrons since they’re almost identical. This kind of bond is called a non-polar bond. Carbon-Carbon and Carbon-Hydrogen bonds (also called Hydrocarbon) is an example on non-polar bonds.
A polar bond occurs when the difference in electronegativity between two atoms are more than or equal to 0.9. In a polar bond, there’s a partial negative charge (δ−) and a partial positive charge (δ+). This is the case in a water molecule H2O. The bond between Oxygen and Hydrogen is polar since difference in electronegativity is 1.2. (Electronegativity of Oxygen is 3.4 and Electronegativity of Hydrogen is 2.2).
- Published in Water Chemistry, Water Treatment
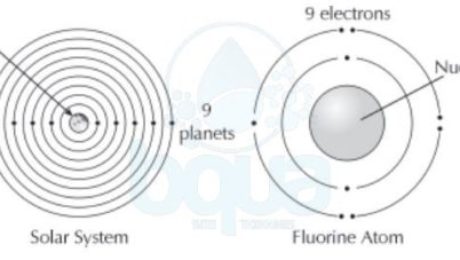
Electron configuration:
The electron configuration is simply the orientation of electrons about the nucleus of the atom. It is known that the atom structure consists electrons, protons and neutrons. An electron has a negative charge, a proton has a positive charge and is in the nucleus with neutrons. Neutrons have neutral charges (non-charged particles).
In a neutral atom, the number of electrons that electron configuration forms are always equal to the number of protons inside the nucleus. The neutron does not contribute to the electrostatic force which is the force that holds the atom together.
It was found that in the atom structure, electrons tend to orient themselves in a particular fashion. This orientation is called the electron configuration. When atoms get larger and gain more electrons, electrons will exist in particular orbits around the nucleus. A living example of such practice is how each planet in our solar system circles the sun in its own orbit.
electron configuration electrons orient themselves around nucleus protons earth planets
In the electron configuration, we find that more than one electron may exist in the same orbit around the nucleus. The first orbital may contain one to two electrons. While the second orbital may contain up to eight electrons. Each subsequent orbital can hold more and more electrons.
Equation for electron configuration
The equation for the number of electrons in each energy level is:
# of electrons = 2(n)^2 Â where “n” is the nth level
For example: Number of electrons in first energy level = 2(1)^2 = 2(1) = 2 electrons
While number of electrons in the second energy level = 2(2)^2 = 2(4) = 8 electrons and so on.
In the electron configuration, an atom is most stable when its outermost orbital is completely full of electrons. In other words two electrons in the first orbital makes the atom more stable than one. While 8 electrons in the second orbital makes it more stable than < 8 electrons.
atom structure more stable when outermost orbital full of electrons
The orbitals are usually illustrated in two dimensional drawings, in reality the orbitals exist in various three dimensional shapes. Each orbital contains one or more suborbitals identified as s, p, and d. The s, p, and d suborbitals are shaped differently. The shape of the suborbital does not affect the number of electrons in the orbital.
- Published in Water Chemistry, Water Treatment
What is Avogadro Number ?
Avogadro Number also known as Avogadro Constant is the number of atoms (6.022 x 1023) in one gram atomic weight (mole) of an element. Or also Avogadro Number can be defined as the number of molecules in a gram molecular weight (mole) of a compound.
It is sometimes given the symbol of NA or L and the unit of measure is mol-1 as per the International System of Units (SI).
Amadeo Avogadro
Avogadro Biography
Full name: Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto, Count of Quaregna and Cerreto. Italian scientist born on the 9th of August 1776 in Turin, Sardinia. Deceased on the 9th of July 1856.
Avogadro 1837 – 1841 published 4 big volumes discussing in details the physics of matter.
Avogadro’s findings and in particular “Avogadro Number” were completely ignored until Stanislao Cannizarro in 1860 presented them at the Karlsruhe Conference. Which was four years after Avogadro left this world. The reason the conference was held is to clarify the confusion that existed at that time about atoms and molecules and their masses.
Still after that Cannizarro presented his findings, not all scientists were convinced. After a decade – with continued strong advocacy from Cannizarro – Avogardo’s hypothesis became more widely accepted and this is when it was called after Avogadro – Avogadro Number.
Today, Avogadro is considered one of the founders of atomic-molecular chemistry
Avogadro Number was first defined and introduced by Jean Baptiste Perrin as the number of atoms in one gram atomic weight of Hydrogen. Which basically means one gram of hydrogen. Later on, Avogadro Number had been redefined as the number of atoms in 12 grams atomic weight of the isotope Carbon-12 (12C). Furthermore, it also relates the amount of a substance to its molecular weight.
| Table shows the value of Avogadro Number  NA in different units |
|---|
| 6.022(74)x1023Â mol-1 |
| 2.731(12)x1026 (lb-mol)-1 |
| 1.7072(77)x1025 (oz-mol)-1 |
- Published in Water Chemistry, Water Treatment
- 1
- 2